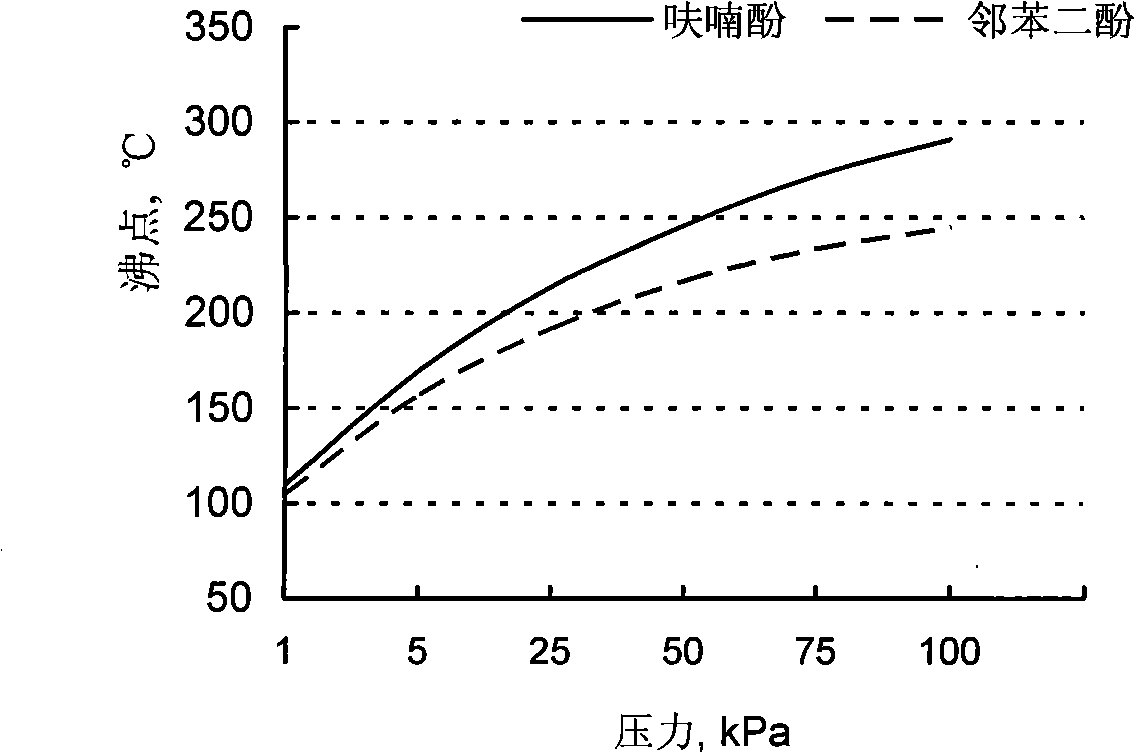
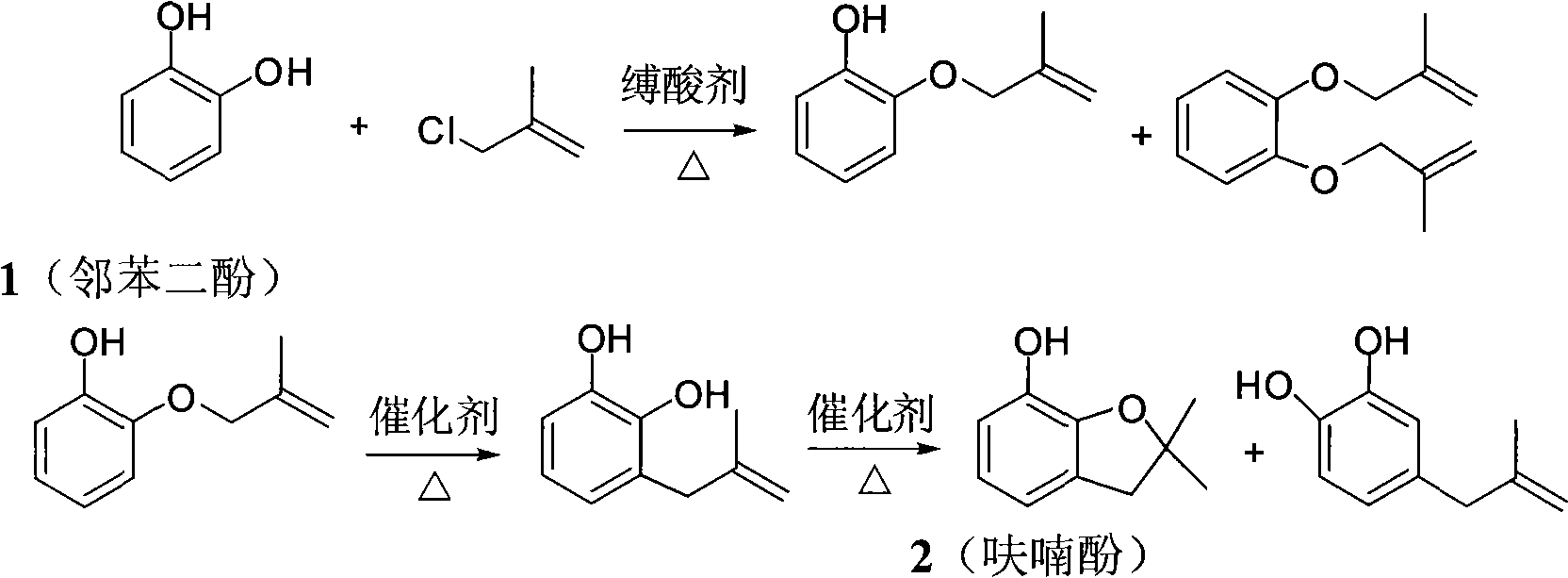
Extraction fractional distillation for separating pyrocatechol in furanol
An extractive distillation method and catechol technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of long operation cycle, unstable operation, increase reflux ratio, etc. The effect of shortened time, convenient operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
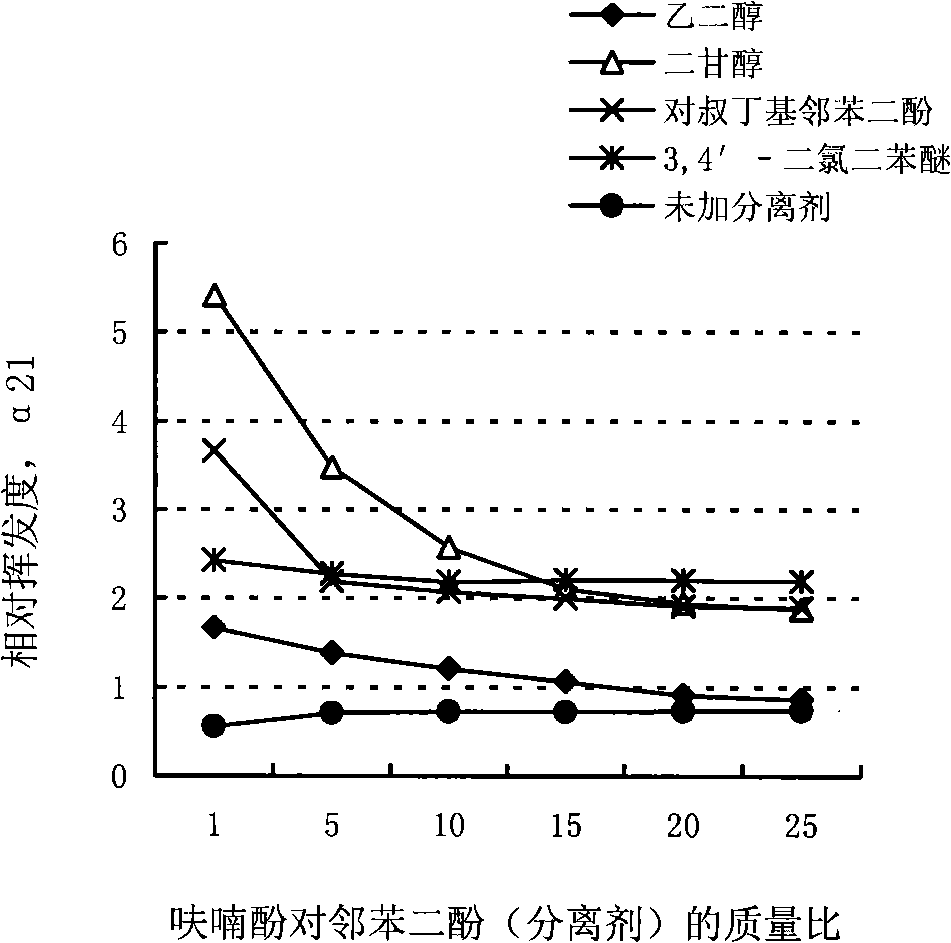
[0030] Add ethylene glycol (glycol), diethylene glycol (diglycol), p-tert-butylcatechol (4-TBC) and 3,4′-diglycol at a certain mass ratio of furanol to catechol and separating agent, respectively. Chlorodiphenyl ether (DCDPE) etc. are used as a separating agent, and no separating agent is added to determine the relative volatility of furanol to catechol α 21 The result is as figure 2 :
[0031] figure 2 It shows that in the absence of separation agent, the relative volatility of furanol to catechol α 21 Both are less than 1, and the average is 0.70. This result shows that the boiling point of furanol is higher than that of catechol. If there is no third component, the fraction extracted from the top of the tower should be high-purity phthalate after sufficient separation in the rectifying tower. diphenols. After adding a certain amount of a certain separating agent, α 21 The relative volatility of furanol and p-catechol can be improved by adding diethylene glycol, p-ter...
Embodiment 2-8
[0033] Taking diethylene glycol as the furan phenol extractive distillation experiment of separating agent, the results are listed in Table 1.
[0034] Table 1 diethylene glycol is the extractive distillation test result of separating agent
[0035]
[0036] The results in Table 1 show that the purity of the main fraction furan phenol collected by conventional rectification without adding a separating agent can only reach between 98.0% and 98.5%, and the single-pass yield of rectification is about 67.0%; The purity of the product fraction furan phenol can reach 99.0%-99.5%, and the single-pass yield of rectification is ≥80.0%.
Embodiment 9-14
[0038] The results of the furan phenol extraction and distillation test using p-tert-butylcatechol as a separating agent are shown in Table 2.
[0039] Table 2 p-tert-butylcatechol is the extractive distillation test result of separating agent
[0040]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com