Method for synthesizing indoline and derivates thereof
A technology of indoline and its derivatives, which is applied in the field of synthesis of indoline and its derivatives, can solve the problems of increasing production costs and expensive copper, and achieve the effects of cost saving, high product yield and easy recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
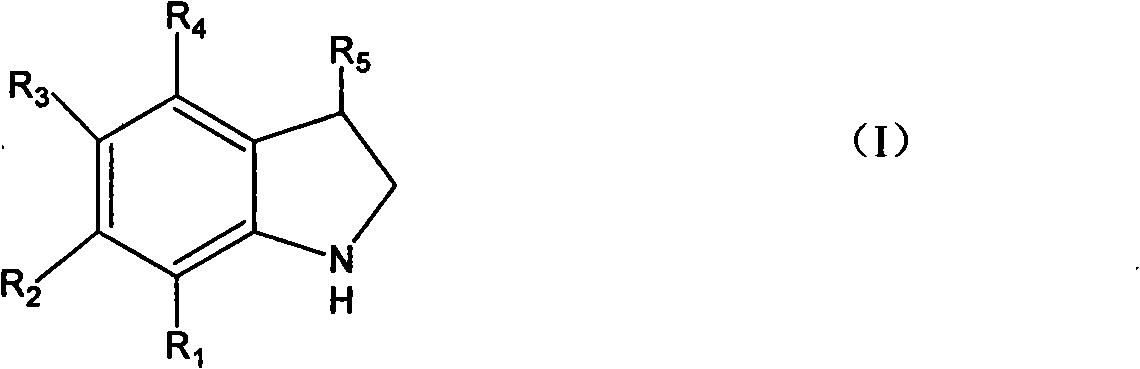
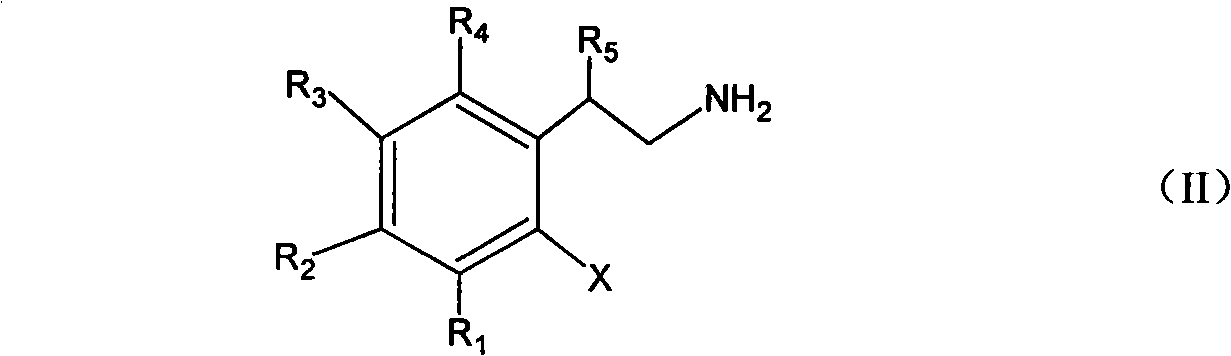
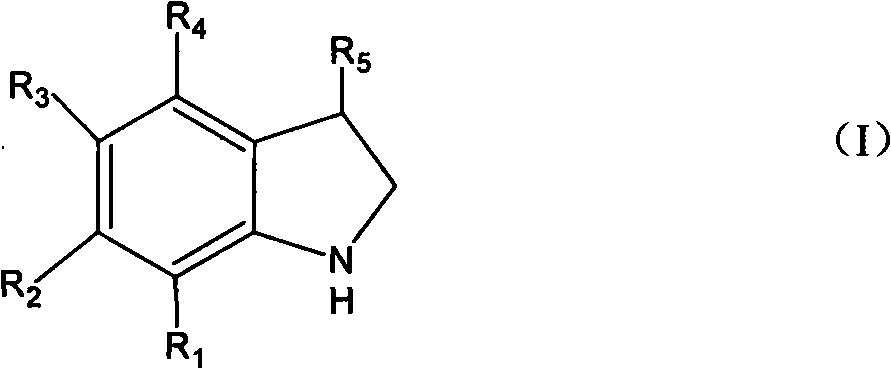
[0031] Embodiment 1 Add the 99% o-chlorophenethylamine of 15.6g (0.10mol) in the flask that electric stirrer, thermometer, condenser tube 100mL are housed, 30g20%NaOH (0.15mol) aqueous solution, the supported copper catalyst of 7.8g ( 10%Cu / TiO 2 ), reacted at 90°C for 8h. The reaction liquid was analyzed by gas chromatography mass spectrometry, and the result was 0.1% o-chlorophenethylamine, 0.8% indole, and 97.8% indoline. Separate the organic layer and the water layer, extract the water layer with 5 mL of chloroform, combine the chloroform layer and the organic layer, pickle with 5% hydrochloric acid, wash with water, and rectify to obtain 11.2 g of indoline (boiling point: 94 ~ 95 ° C / 11 mmHg). The yield is 94.0%, and the gas phase purity is 99.5%.
Embodiment 2
[0032] Embodiment 2 changed o-chlorophenethylamine into 21.4g (0.10mol) o-bromophenethylamine, and others were the same as in Example 1 to obtain 11.4g of product indoline with a yield of 95.7% and a gas phase purity of 99.6%.
Embodiment 3
[0033] In Example 3, the reaction time was changed to 5h, and the others were the same as in Example 1. The results were 1.5% o-chlorophenethylamine, 0.6% indole, 96.9% indoline, 92.5% yield, and 99.4% gas phase purity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com