Stable perindopril tert-butylamine salt tablets and preparation method thereof
A technology of tert-butylamine salt tablets and perindopril, which is applied in the field of medicine, can solve the problems of accelerated degradation, no data showing the stability of the preparation, and stability has not been discussed, so as to achieve the effect of enhancing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
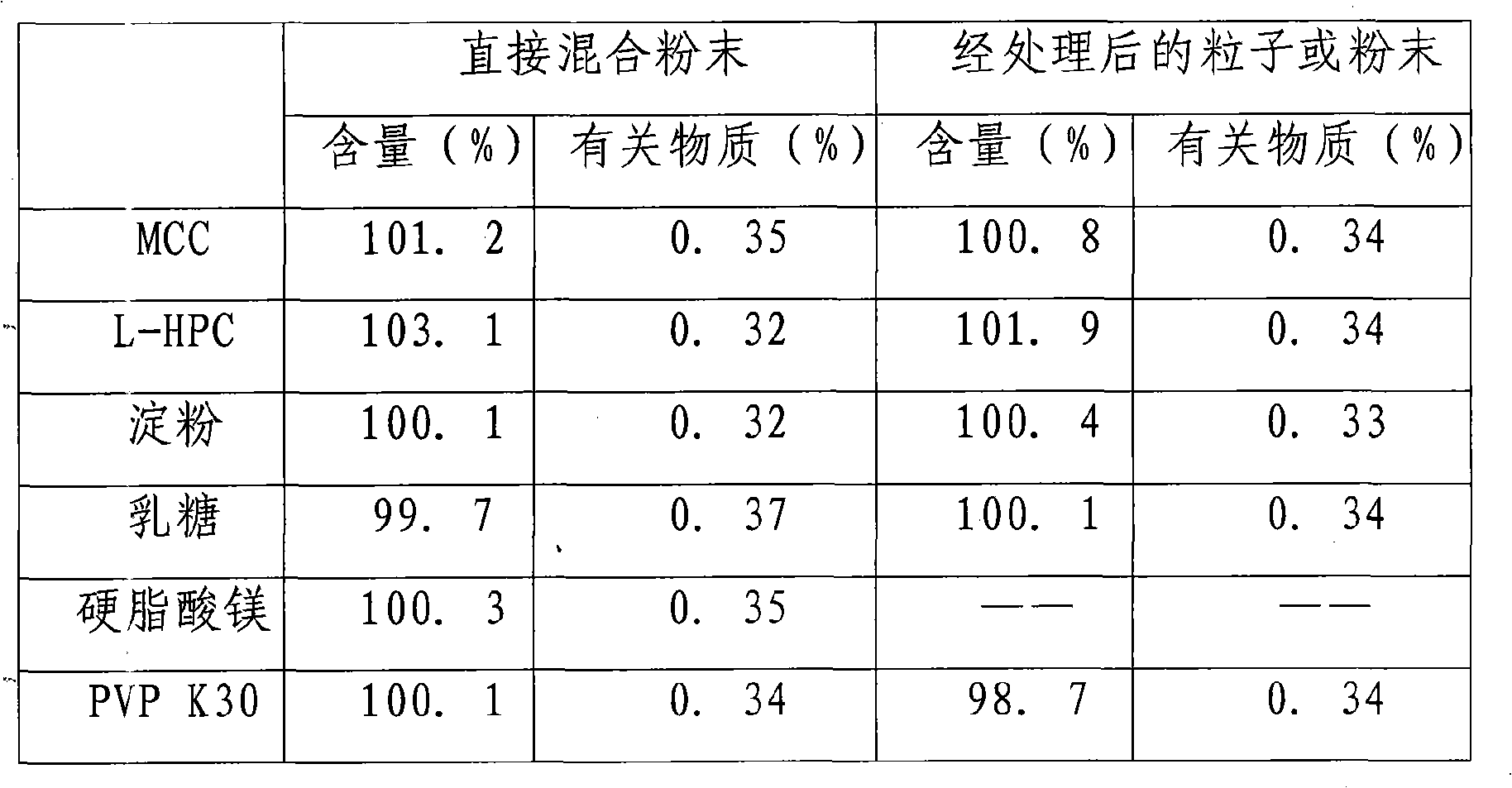
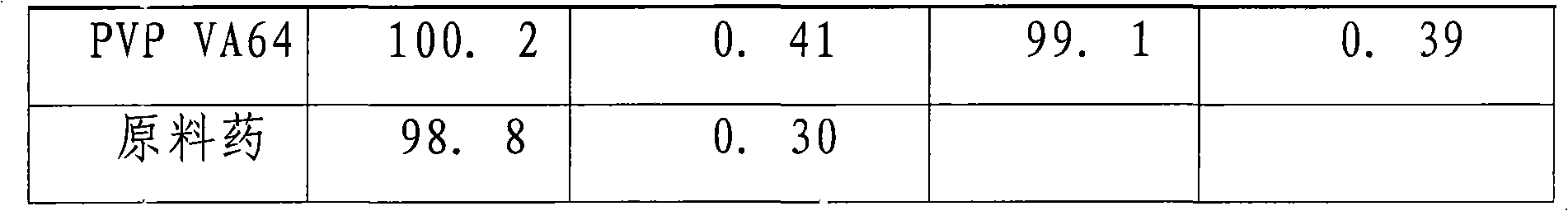
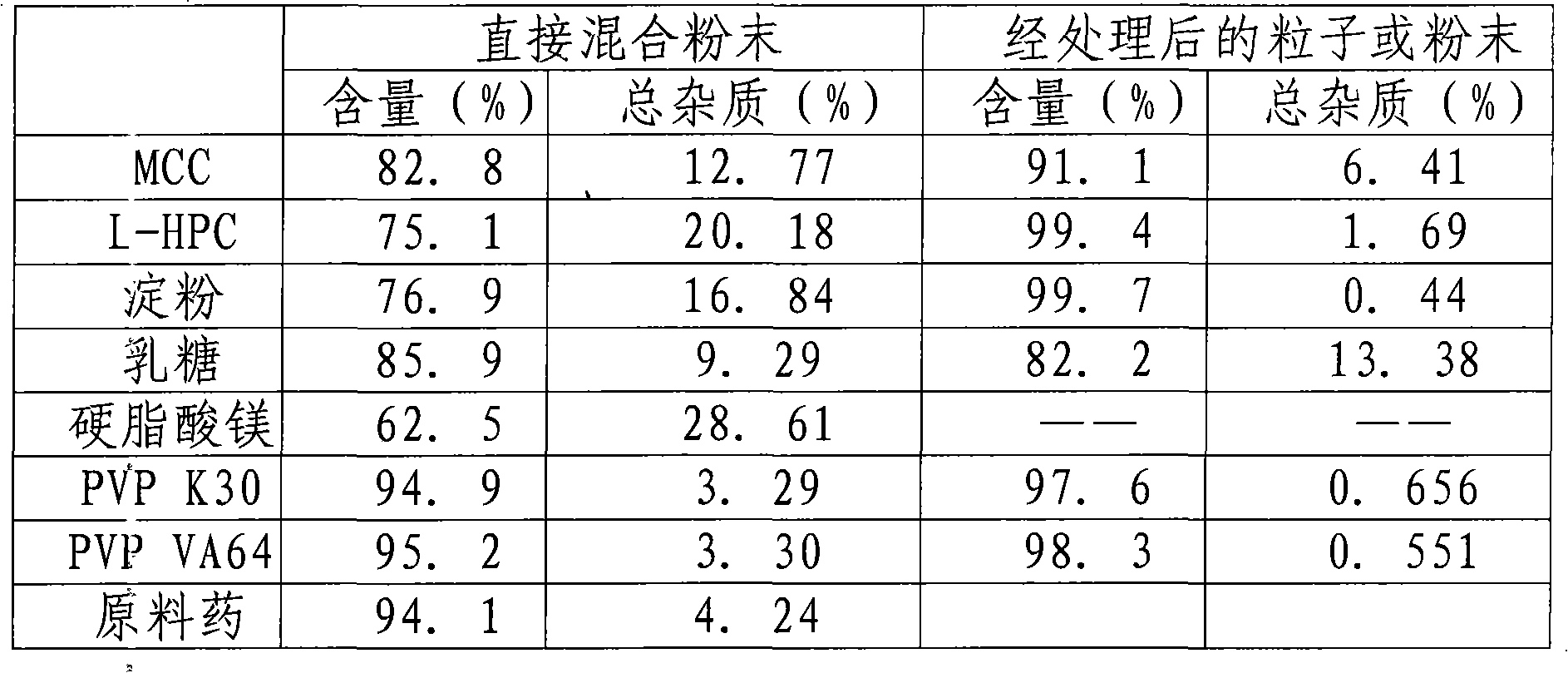
Image
Examples
Embodiment 1
[0047] MCC 30g
[0048] Lactose 30.5g
[0049] Starch 30g
[0050] L-HPC 7g
[0051] Perindopril tert-butylamine salt 2g
[0052] 35% ethanol appropriate amount
[0053] Magnesium stearate 0.5g
[0054]
[0055] 1000 pieces
[0056] Mix MCC, lactose, starch, perindopril tert-butylamine salt, and L-HPC evenly, use 35% ethanol to make a soft material, granulate with a 40-mesh sieve, dry at 40°C, granulate with a 30-mesh sieve, and add stearic acid After the magnesium is mixed evenly, the tablets are obtained.
Embodiment 2
[0058] MCC 30g
[0059] Lactose 34.5g
[0060] Starch 20g
[0061] PVP K30 3g
[0062] L-HPC 10g
[0063] Perindopril tert-butylamine salt 2g
[0064] 50% ethanol appropriate amount
[0065] Magnesium stearate 0.5g
[0066]
[0067] 1000 pieces
[0068] Mix MCC, lactose, starch, PVP K30, L-HPC, perindopril tert-butylamine salt evenly, use 50% ethanol to make soft material, granulate with 40 mesh sieve, dry at 40℃, granulate with 30 mesh sieve, add hard After the magnesium fatty acid is mixed evenly, it can be obtained by pressing into tablets.
Embodiment 3
[0070] MCC 30g
[0071] Lactose 34.5g
[0072] Starch 20g
[0073] L-HPC 10g
[0074] Perindopril tert-butylamine salt 2g
[0075] 5% (50%) ethanol solution of PVP K30 appropriate amount
[0077]
[0078] 1000 pieces
[0079] Mix MCC, lactose, starch, L-HPC, and perindopril tert-butylamine salt evenly, use 5% PVPK30 solution to make soft material, sieve 40 mesh, dry at 40°C, sieve 30 mesh, add stearic acid After the magnesium is evenly mixed, it is compressed into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com