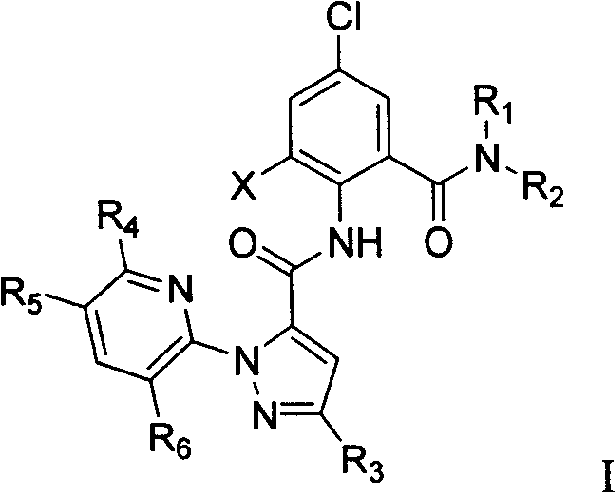
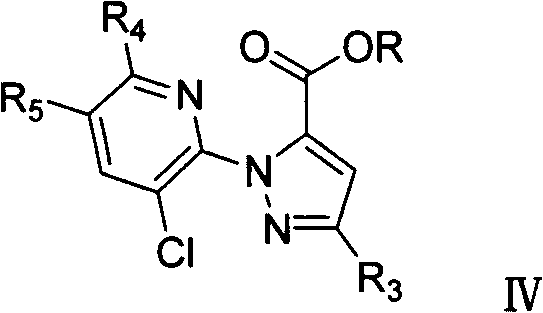
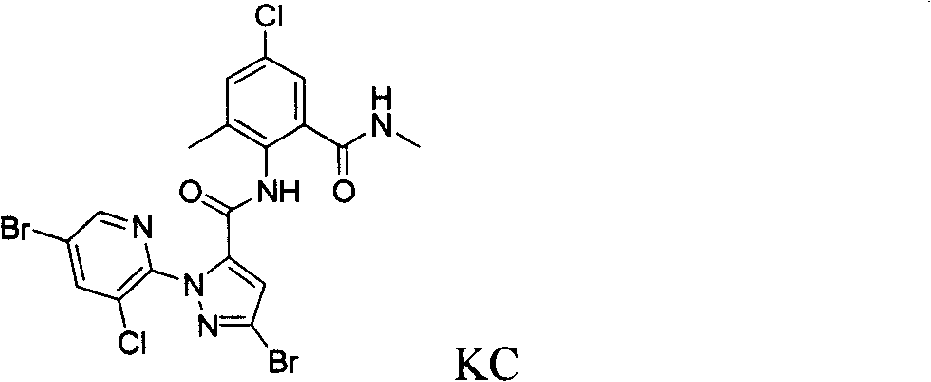
1-substituted pyridyl-pyrazol acid amide compounds and use thereof
A technology for pyrazole amides and compounds, which is applied in the field of 1-substituted pyridyl-pyrazole amides, and can solve the problems that 1-substituted pyridyl-pyrazole amides have no insecticidal and bactericidal activities.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0131] Example 1, the preparation of compound 1.2
[0132] (1) Preparation of 2,4-dicarbonyl pentanoic acid methyl ester
[0133]
[0134] Add 25% methanol solution of sodium methylate (43.2 grams, 0.200 moles), 200 milliliters of methanol in the reaction flask, add dimethyl oxalate (23.6 grams, 0.200 moles) and acetone (11.6 grams, 0.200 moles) dropwise under ice-salt bath moles), the reaction system was maintained at 0-5°C and stirred for 8 hours. The reaction solution was poured into 200 ml of water, extracted with 150 ml of ethyl acetate to remove organic impurities, kept the inorganic phase, adjusted to pH 2-3 with concentrated hydrochloric acid, extracted with 3×200 ml of ethyl acetate, and the organic phase was washed with water, After washing with saturated brine, drying with anhydrous magnesium sulfate, and concentrating to obtain 25.9 g of yellow oil, the yield is 90%.
[0135] 1 H NMR (300MHz, CDCl 3 ): 6.396 (s, 1H, enol form), 3.906 (s, 3H), 2.279 (s, 3H). ...
example 2
[0153] Example 2, the preparation of compound 1.11
[0154] (1), 3,5, the synthesis of 6-trichloro-2-hydrazinopyridine
[0155]
[0156] Add 2,3,4,5-tetrachloropyridine (10 g, 46 mmol), 80% hydrazine hydrate (14 g, 230 mmol) and 100 milliliters of dioxane successively in the reaction flask, and stir at reflux temperature 20 hours. The reaction solution was cooled overnight, white crystals were precipitated, filtered and dried to obtain 6.1 g of solid, yield: 63%, melting point: 187-189°C.
[0157] 1 H NMR (300MHz, CDCl 3 ): 7.553 (s, 1H), 6.347 (br s, 1H), 3.927 (br s, 2H).
[0158] (2), the synthesis of 1-(3,5,6-trichloropyridin-2-yl)-5-(2-furyl)-3-trifluoromethyl-1H-pyrazole
[0159]
[0160] Add 4,4,4-trifluoro-1-(2-furyl)butane-1,3-dione (10 g, 48 mmol), 3,5,6-trichloro-2 -Hydrazinopyridine (10 g, 48 mmol), add glacial acetic acid (100 ml), heat to reflux, the reaction is complete, evaporate the solvent under reduced pressure, add ethyl acetate (300 ml), and se...
example 3
[0172] Example 3, the preparation of compound 1.17
[0173](1), 3, the synthesis of 5-dichloro-2-hydrazinopyridine
[0174]
[0175] 2,3,5-Trichloropyridine (20 g, 0.11 mol), 80% hydrazine hydrate (34 g, 0.55 mol) and 200 ml of dioxane were successively added to the reaction flask, and stirred at reflux temperature for 20 hours. The reaction solution was cooled overnight, white crystals were precipitated, filtered, and dried to obtain 14 g of solid, yield: 72%, melting point: 187-189°C.
[0176] 1 H NMR (300MHz, DMSO-d 6 ): 8.123(d, 1H), 7.849(d, 1H).
[0177] (2), the synthesis of 1-(3,5-dichloropyridin-2-yl)-3-pyrazolidinone-5-carboxylic acid ethyl ester
[0178]
[0179] Add 300 milliliters of absolute ethanol and sodium ethylate (4.1 grams, 61 mmoles), 3,5-dichloro-2-hydrazinopyridine (10 grams, 56 mmoles) in the reaction bottle, the mixture is heated to reflux for 5 minutes, drop Add diethyl maleate (10 g, 61 mmol). Heating to reflux was continued for 10 minut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com