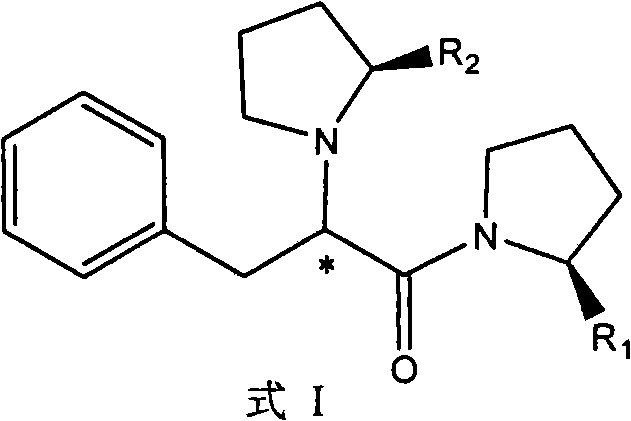
Phenylalanyl pyrrolidine derivates, preparation method and application thereof
A technology of phenylpropionylpyrrolidine and pyrrolidine, applied in the field of medicinal chemistry, can solve the problems of impaired secretion, insufficient secretion level of GLP-1 and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: 2-(S)-(2′-(S)-formamide)pyrrolidine-phenylpropionyl-(2′-(S)-cyano)pyrrolidine
[0040]
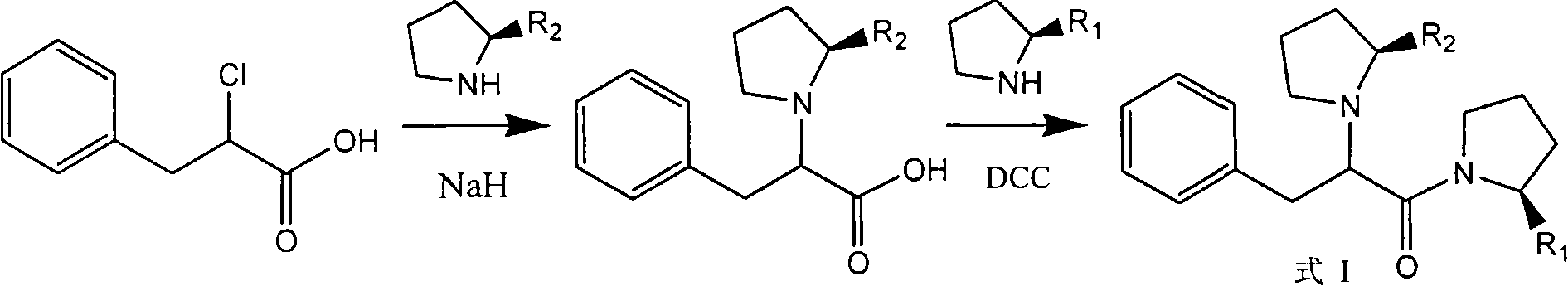
[0041] Step 1: 2-(R)-Chlorophenylpropionyl(2′-(S)-carboxamide)pyrrolidine
[0042] 61 g of 2-(R)-chlorophenylpropionyl chloride (0.3 mol) was dissolved in 20 ml of anhydrous tetrahydrofuran solution to prepare a tetrahydrofuran solution of 2-(R)-chlorophenylpropionyl chloride. Another 37.6 grams of L-proline amide (0.33mol), 55 grams of potassium carbonate (0.4mol) and 180 milliliters of anhydrous tetrahydrofuran solution were added to a 500 milliliter reaction flask, and 2-(R)-chlorine was added dropwise below 10°C. The tetrahydrofuran solution of phenylpropanoyl chloride was reacted at room temperature for 3 hours after the dropwise addition was completed. After the reaction was completed, the reaction solution was filtered, the filtrate was evaporated under reduced pressure to remove tetrahydrofuran, the residue was poured into 200 ml of ice water, and the solid wa...
Embodiment 2
[0047] Example 2: 2-(S)-pyrrolidine-phenylpropionyl-(2′-(S)-formamide)pyrrolidine
[0048]
[0049] Take 14 grams (0.05mol) of the product obtained in step 1 in Example 1 and dissolve it in 30% anhydrous tetrahydrofuran solution to prepare 2-(R)-chlorophenylpropionyl-(2'-(S)-formamide)- solution of pyrrolidine in tetrahydrofuran. Dissolve 4.6 g of pyrrolidine (0.065 mol) in 100 ml of anhydrous tetrahydrofuran, cool in an ice bath to below 0°C, add 2.6 g of 60% sodium hydride (0.065mol) in batches, control the internal temperature not to exceed 5°C, and continue The reaction was stirred for 1 hour. Add 2-(R)-chlorophenylpropionyl-(2′-(S)-formamide)-pyrrolidine in tetrahydrofuran dropwise below 5°C. After the dropwise addition is complete, stir the reaction at room temperature for 1 hour. The solvent was evaporated under reduced pressure, the residue was poured into 100 g of ice cubes, the solid was precipitated, filtered, and washed with water to obtain an off-white powder...
Embodiment 3
[0050] Example 3: 2-(S)-(2′-(S)-formic acid)pyrrolidine-phenylpropionyl-(2′-(S)-formic acid)pyrrolidine
[0051]
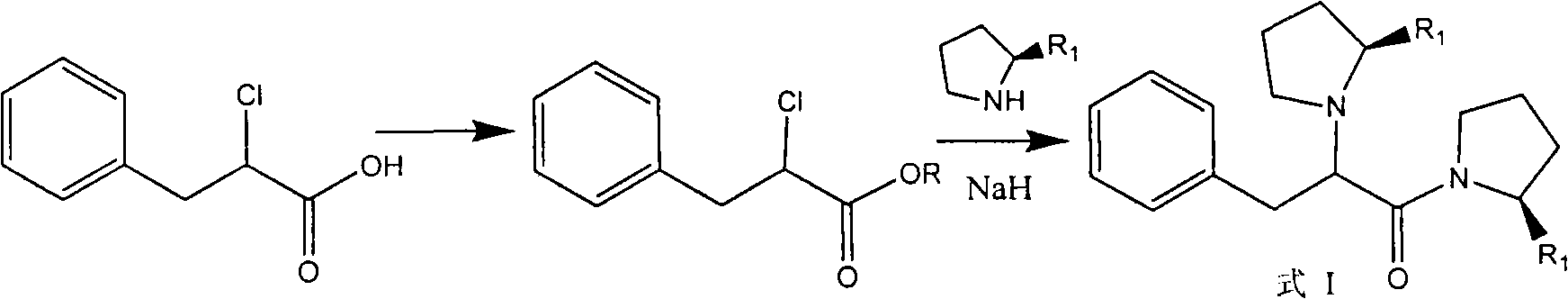
[0052] 17 grams (0.075mol) of the product obtained in step 2 of Example 1 was dissolved in 30% anhydrous tetrahydrofuran solution to prepare 2-(R)-chlorophenylpropionyl-(2'-(S)-cyano)-pyrrole alkanes in tetrahydrofuran. Dissolve 9.8 g of L-proline (0.085 mol) in 100 ml of anhydrous tetrahydrofuran, cool in an ice bath to below 0°C, add 6.8 g of 60% sodium hydride (0.17 mol) in batches, and control the internal temperature not to exceed 5 °C, stirred for 1 hour. Below 5°C, 2-(R)-chlorophenylpropionyl-(2′-(S)-cyano)-pyrrolidine tetrahydrofuran solution was added dropwise, and after the dropwise addition was completed, the reaction was stirred at room temperature for 2 hours. The solvent was evaporated under reduced pressure, the residue was poured into 100 g of ice cubes, the solid was precipitated, filtered, and washed with water to obtain a light yellow powde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com