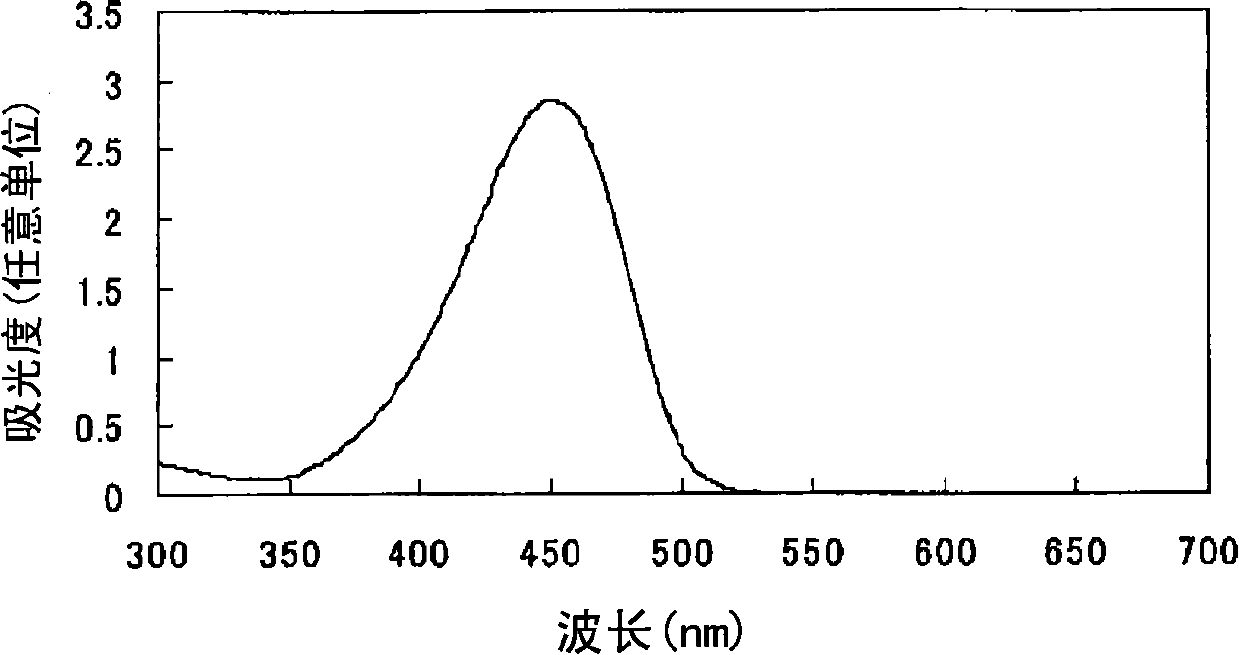
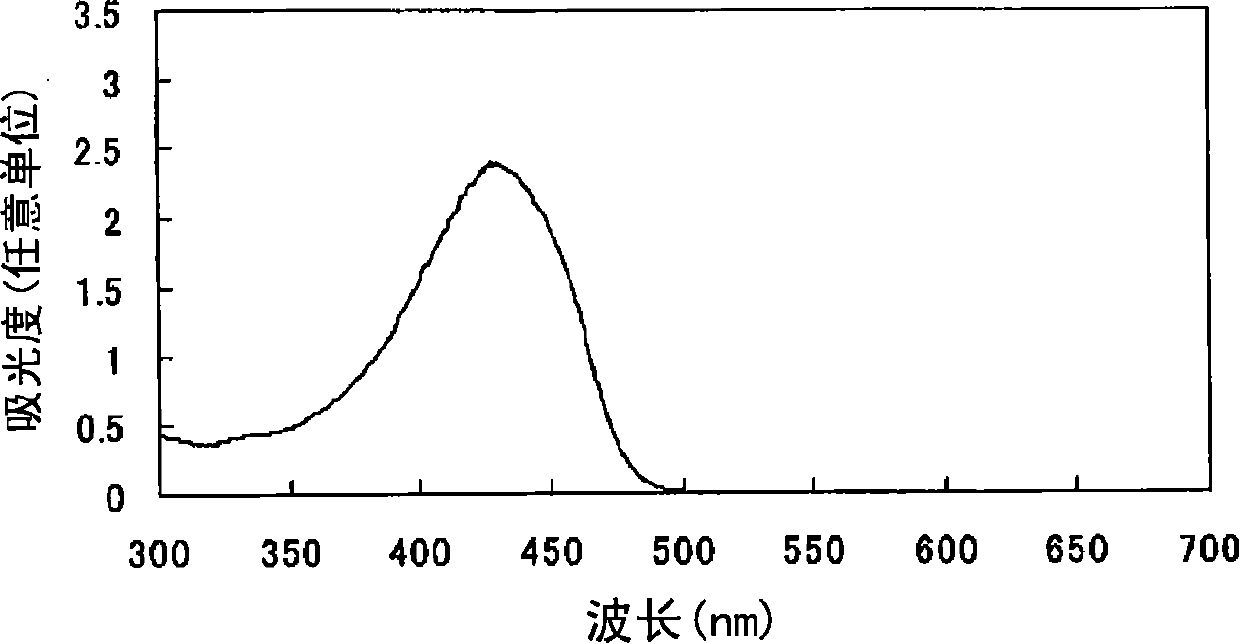
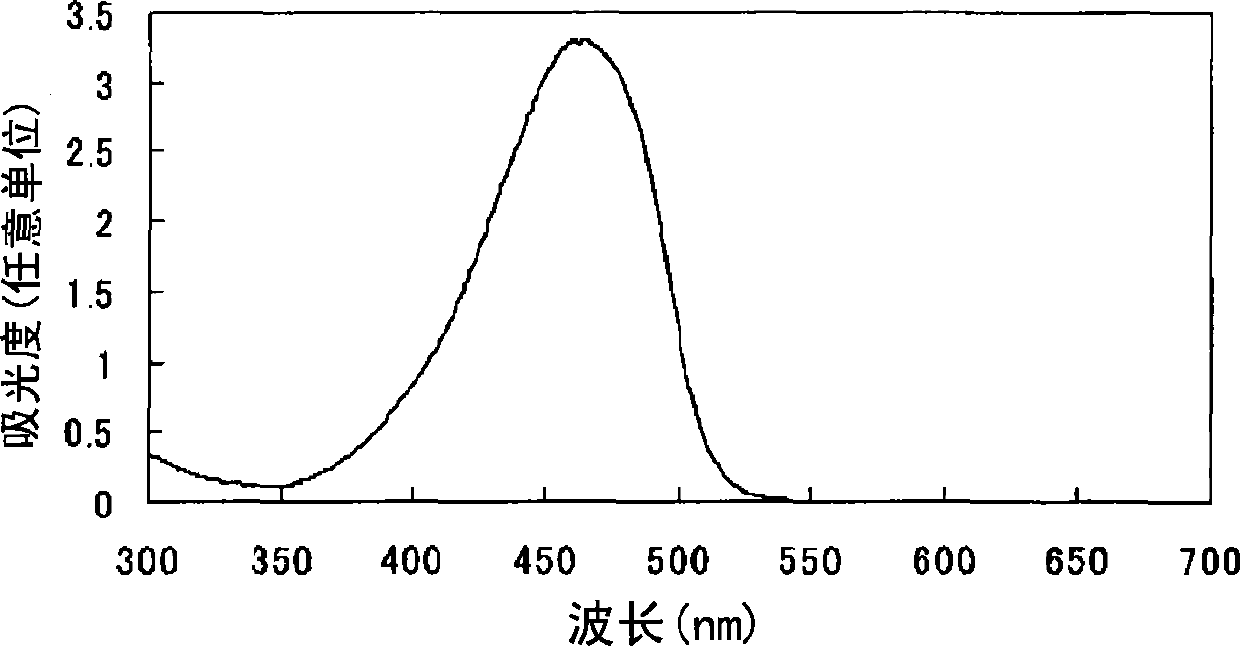
Azo compound or salts thereof
An azo compound, R13 technology, applied in the direction of azo dyes, monoazo dyes, disazo dyes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] After adding 100 parts of water to 10 parts of 2,2'-benzidine disulfonic acid (water content 30%) represented by formula (a-1), the pH was adjusted to 7-8 with 30% sodium hydroxide aqueous solution under ice cooling. The following operations were carried out under ice cooling. Add 5.6 parts of sodium nitrite and stir for 30 minutes. 14.8 parts of 35% hydrochloric acid was added a little at a time to form a brown solution and stirred for 2 hours. Dissolve 3.8 parts of sulfamic acid in 38.3 parts of water to form an aqueous solution, add the aqueous solution to the reaction solution, and stir to obtain a suspension containing diazonium salt.
[0070]
[0071] After adding 47.9 parts of water to 9.6 parts of 1-ethyl-3-carbamoyl-4-methyl-6-hydroxypyridin-2-one represented by formula (c-1), use 30% sodium hydroxide to The aqueous solution adjusts the pH to 8-9.
[0072]
[0073] The following operations were performed under ice-cooling. The above-mentioned alkaline...
Embodiment 2
[0080] After adding 156 parts of ethanol and 52 parts of water to 10.4 parts of 2,2'-bis(trifluoromethyl)benzidine represented by formula (a-2), the pH was adjusted to 7 with 30% aqueous sodium hydroxide solution under ice cooling. ~8. The following operations were carried out under ice cooling. Add 6.7 parts of sodium nitrite and stir for 30 minutes. 30.4 parts of 35% hydrochloric acid was added a little at a time to form a brown solution and stirred for 2 hours. Dissolve 3.1 parts of sulfamic acid in 31 parts of water to form an aqueous solution, add the aqueous solution to the reaction solution, and stir to obtain a suspension containing diazonium salt.
[0081]
[0082]After adding 15 parts of ethanol and 221 parts of water to 14.7 parts of 1-ethyl-3-carbamoyl-4-methyl-6-hydroxypyridin-2-one represented by formula (c-1), use 30% Sodium hydroxide aqueous solution adjusts the pH to 8-9.
[0083] The following operations were performed under ice-cooling. The above-men...
Embodiment 3
[0087] After adding 1000 parts of water to 100 parts of 2,2'-benzidine disulfonic acid (water content 30%) represented by formula (a-1), the pH was adjusted to 7-8 with 30% aqueous sodium hydroxide solution under ice cooling. The following operations were carried out under ice cooling. Add 56.1 parts of sodium nitrite and stir for 30 minutes. 148 parts of 35% hydrochloric acid were added a little at a time to form a brown solution and stirred for 2 hours. Dissolve 38.3 parts of sulfamic acid in 383 parts of water to form an aqueous solution, add the aqueous solution to the reaction solution, and stir to obtain a suspension containing diazonium salt.
[0088] After adding 761 parts of water to 76.1 parts of 1-ethyl-3-carbamoyl-4-methyl-6-hydroxypyridin-2-one represented by formula (c-2), use 30% sodium hydroxide under ice-cooling The aqueous solution adjusts the pH to 8-9.
[0089]
[0090] The following operations were performed under ice-cooling. The above-mentioned al...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com