2-amido-4, 5-diaryl miazines compound, preparation and pharmaceutical use thereof
A technique for diarylpyrimidines and compounds, which is applied in the field of heterocyclic compounds and can solve problems such as weak effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of compound 1
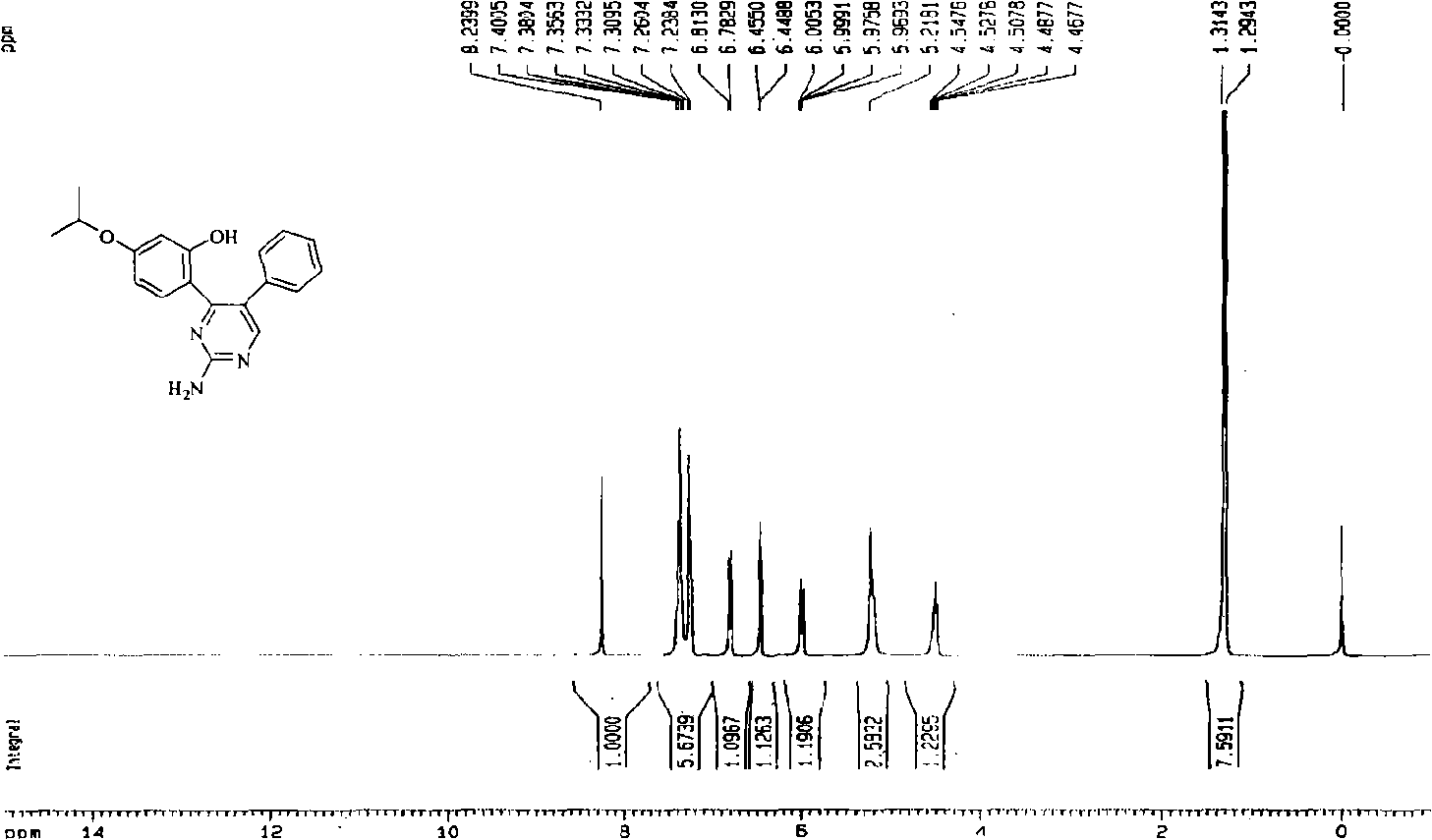
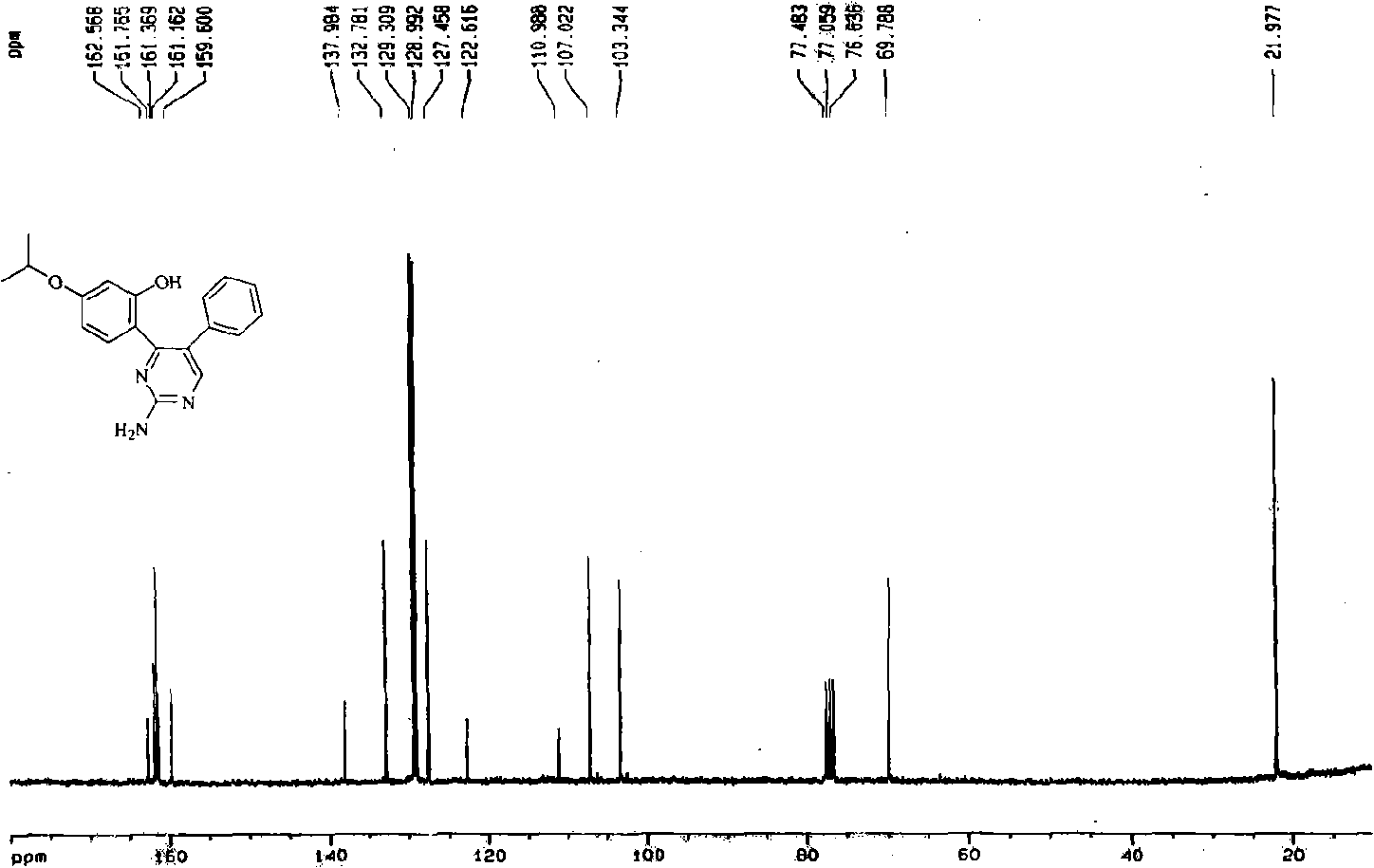
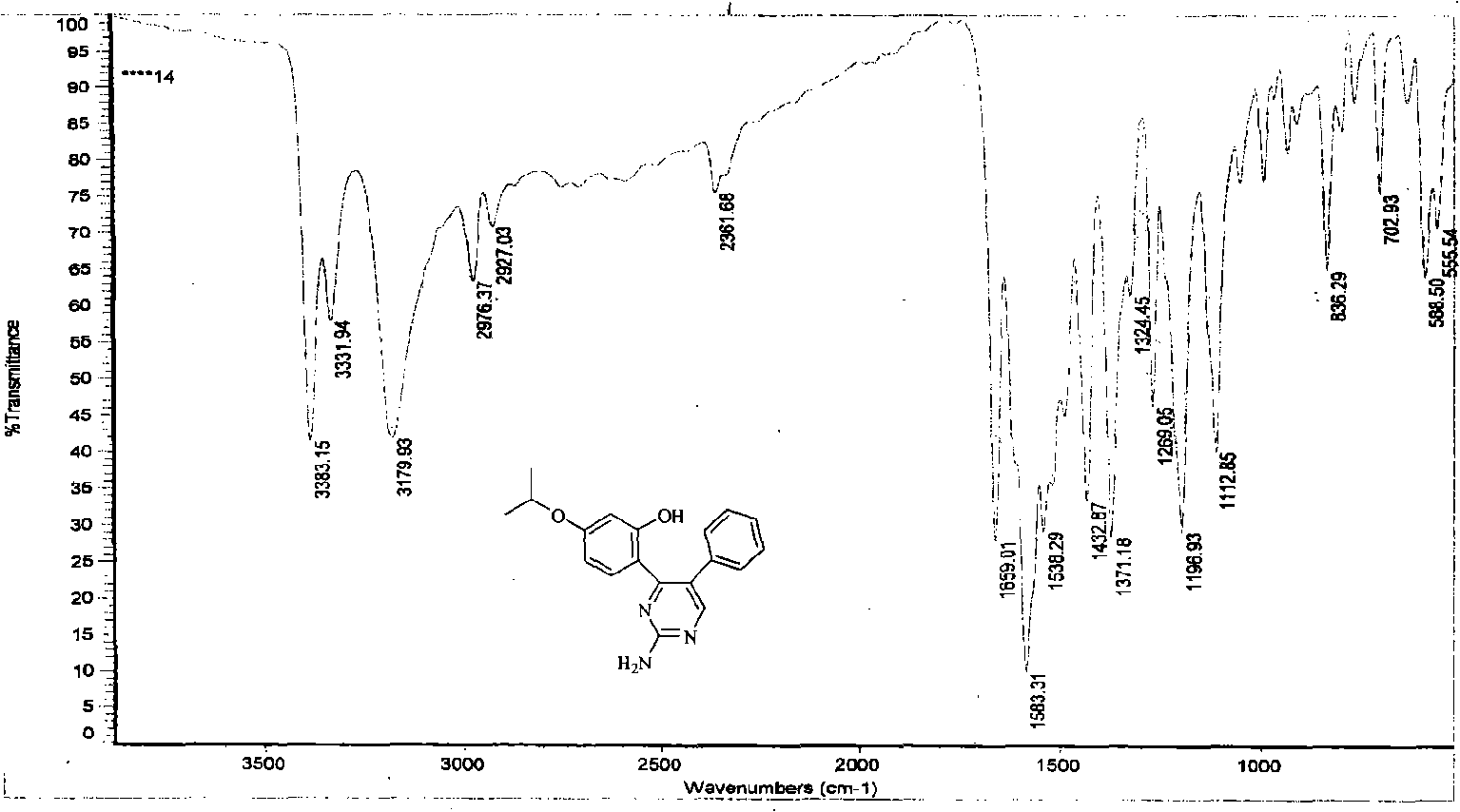
[0038] In this example, first add 7-isopropoxy isoflavone into the reaction kettle, add 100 times the weight of ethanol of 7-isopropoxy isoflavone compound under stirring with a mixer, then add guanidine, 7-isopropoxy The molar ratio of 8-yl isoflavone to guanidine is 1:4, and the temperature of the reaction solution is 80°C with a temperature regulating device. During the reaction, 10% NaOH aqueous solution is continuously added dropwise to keep the pH of the reaction system=8-10, and react for 9 hours , to obtain a mixture of 2-amino-4-(2-hydroxyl-4-isopropoxyphenyl)-5-phenylpyrimidine and unreacted raw materials, the reacted mixture was distilled to dryness under reduced pressure, and then added to the mixture 80 times 3% HCl aqueous solution by weight, heated, dissolved, filtered, adjusted to neutral, left to stand overnight to form a precipitate, filtered under reduced pressure to obtain 2-amino-4-(2-hydroxyl-4-isopropoxyphenyl)- Crude ...
Embodiment 2
[0134] Preparation of Compound 1
[0135] In this example, first add 7-isopropoxy isoflavone into the reaction kettle, add 180 times the weight of 7-isopropoxy isoflavone compound toluene under stirring with a mixer, then add guanidine, 7-isopropoxy The molar ratio of 8-yl isoflavone to guanidine is 1:4, and the temperature of the reaction solution is 100°C with a temperature regulating device. During the reaction, 12% NaOH aqueous solution is continuously added dropwise to keep the pH of the reaction system=8-10, and the reaction is 5 hours , to obtain a mixture of 2-amino-4-(2-hydroxyl-4-isopropoxyphenyl)-5-phenylpyrimidine and unreacted raw materials, the reacted mixture was distilled to dryness under reduced pressure, and the mixture was added 50 times 5% HCl aqueous solution by weight, heated, dissolved, filtered, adjusted to neutral, left standing overnight, a precipitate was formed, filtered under reduced pressure to obtain 2-amino-4-(2-hydroxyl-4-isopropoxyphenyl) - C...
Embodiment 3
[0139] Preparation of Compound 1
[0140] In this example, first add 7-isopropoxy isoflavone into the reaction kettle, add 80 times the weight of methanol of 7-isopropoxy isoflavone compound under stirring with a mixer, then add guanidine, 7-isopropoxy The molar ratio of 8-based isoflavone to guanidine is 1:1, and the temperature of the reaction solution is 50°C with a temperature regulating device. During the reaction, 5% KOH aqueous solution is continuously added dropwise to keep the pH of the reaction system = 8-10, and the reaction is carried out for 30 hours , to obtain a mixture of 2-amino-4-(2-hydroxyl-4-isopropoxyphenyl)-5-phenylpyrimidine and unreacted raw materials, the reacted mixture was distilled to dryness under reduced pressure, and then added to the mixture 110 times 2% HCl aqueous solution by weight, heated, dissolved, filtered, adjusted to neutral, left to stand overnight to form a precipitate, filtered under reduced pressure to obtain 2-amino-4-(2-hydroxyl-4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com