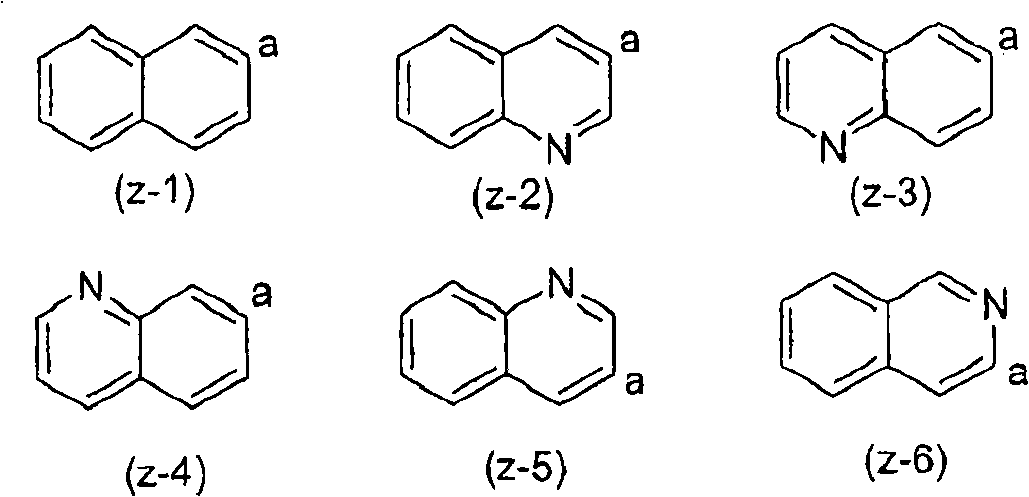
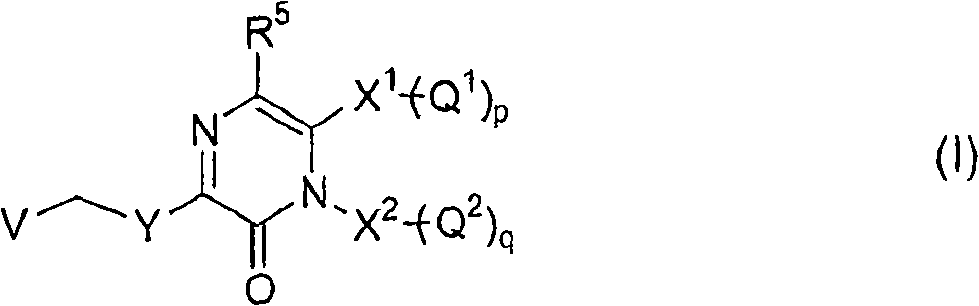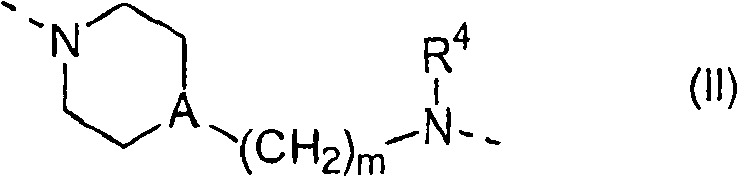Substituted pyrazinone derivatives as alpha2c-adrenoreceptor antagonists
A technology of pyrazinone and derivatives, applied in the field of substituted pyrazinone derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0112] Preparation of intermediate compound (1-4)
[0113]
[0114] Option 1B
[0115] Alkylation reactions of raw material 2,3-dichloropyrazine (I-1) and amino derivatives (I-2) (Scheme 1A) or (I-5) (Scheme 1B) alkylation reaction can be in the proton In an inert solvent such as DMF or DMSO, in an inorganic base such as K 2 CO 3 、Na 2 CO 3 , NaOH or KOH, at normal temperature, or by conventional heating or microwave radiation for a period of time to ensure that the reaction is complete. Under conventional heating, the reaction can usually be carried out for about 16 hours.
[0116]The hydrolysis reaction can be carried out in an acidic inorganic solvent such as 10% HCI in water, using a co-solvent such as THF, by conventional heating or microwave heating for a period of time to ensure the reaction is complete, and the reaction can usually be carried out for about 16 hours under conventional heating, or Under basic conditions such as NaOH aqueous solution or in DMSO ...
Embodiment C1
[0370] Example C.1: α 2C - Adrenergic receptor binding assay
[0371] Cell culture and plasma membrane preparation
[0372] will be stably transfected with human epinephrine-α 2A , -α 2B or alpha 2C CHO cells with recipient cDNA were cultured in Dulbecco's Modified Eagle's Medium (DMEM) / Ham's F12 nutrient mixture (ratio 1:1) (Gibco, Gent-Belgium) supplemented with 10% heat-inactivated Fetal bovine serum (Life Technologies, Merelbeke-Belgium) and antibiotics (100 IU / ml penicillin G, 100 μg / ml streptomycin sulfate, 110 μg / ml pyruvate and 100 μg / ml L-glutamine). The day before harvest, cells were induced with 5 mM sodium butyrate. After reaching 80-90% confluence, the cells were taken out and added to Ca-free 2+ and Mg 2+ Cells were harvested by centrifugation at 1500xg for 10 minutes in phosphate-buffered saline. Cells were homogenized in Tris-HCl 50 mM using an Ultraturrax homogenizer and centrifuged at 23,500 xg for 10 minutes. The pellet was washed once by re...
Embodiment D1
[0393] Example D.1: Oral drops
[0394] Dissolve 500 g a.i. in 0.5 L 2-hydroxypropionic acid and 1.5 L polyethylene glycol at 60-80°C. After cooling to 30-40°C, 35 L of polyethylene glycol was added and the mixture was stirred well. Then add 1750 g of sodium saccharin to 2.5 L of pure water and add 2.5 L of cola essence and appropriate amount of polyethylene glycol to 50 L under stirring to obtain an oral drop solution containing 10 mg / ml a.i. Fill the resulting solution into suitable containers.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com