Vascular endothelial cells, and preparation and use thereof
A vascular endothelial and cell technology, applied in the field of vascular endothelial cells and their preparation, can solve the problems of easy damage to endothelial cells and difficulty in controlling strength, and achieve the effect of vigorous proliferation in vitro, high purity, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1, separation and cultivation of endothelial cells
[0031] In this experiment, endothelial cells were isolated from fresh umbilical cord veins of healthy full-term neonates. When operating, first cut off the fresh umbilical cord of the newborn.
[0032] The raw materials used in this experiment are as follows: type II collagenase (Gibco), IMDM medium (Gibco, Cat.No.12200-036)), fetal bovine serum (Hyclone), horse serum (Gibco), bovine serum albumin BSA (Fluka), vascular endothelial growth factor VEGF (Prepro Tech), basic fibroblast growth factor bFGF (Prepro Tech), heparin sodium (Shanghai Sinopharm Chemical Reagent Co., Ltd.).
[0033] Type II collagenase solution is prepared by dissolving type II collagenase dry powder in IMDM medium.
[0034] 1. Isolation and cultivation under condition I
[0035] The condition I is as follows: the initial concentration of the type II collagenase solution used is 342U / ml, digested for 10 minutes; the cell culture condi...
Embodiment 2
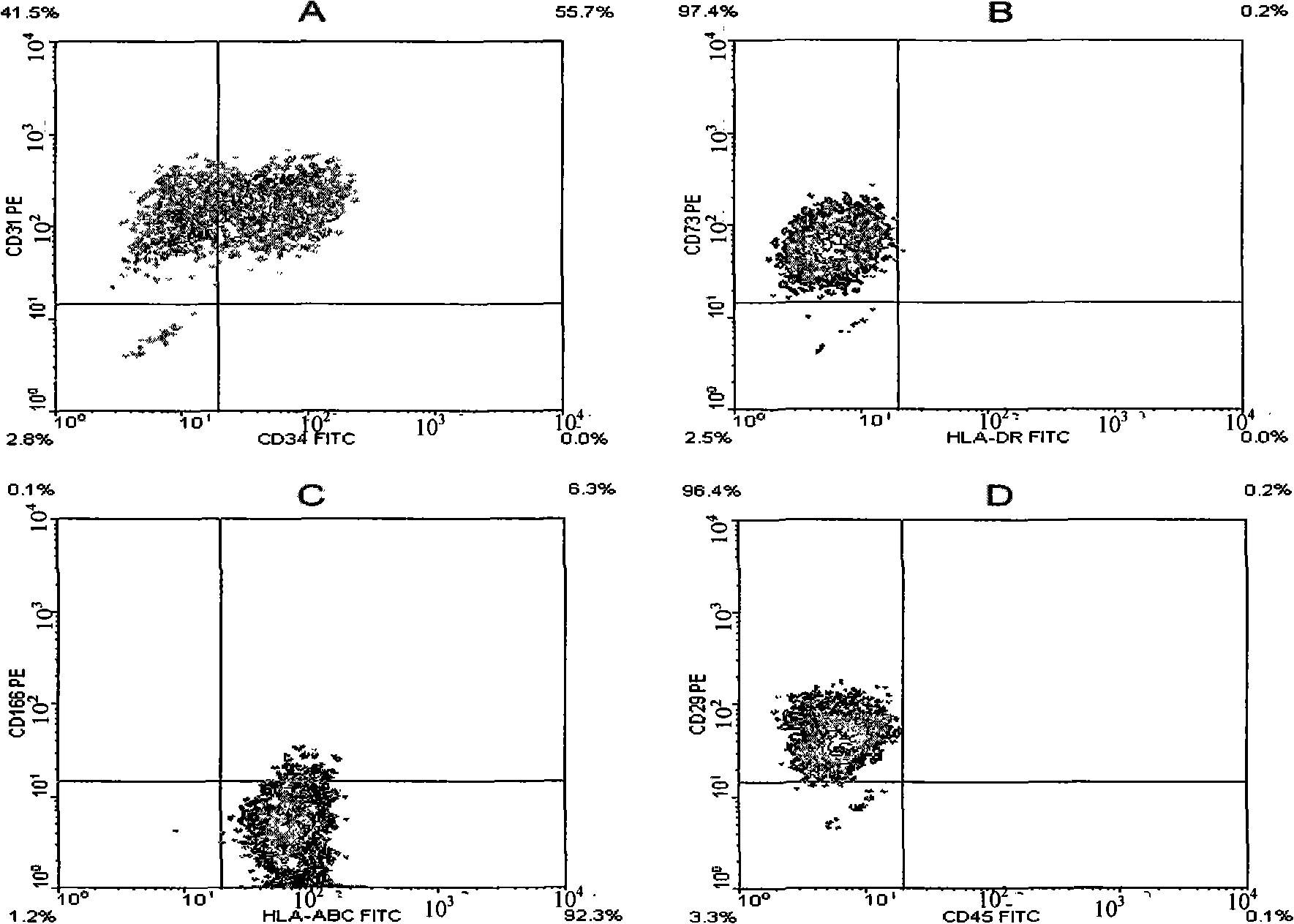
[0052] Embodiment 2, identification of endothelial cells
[0053] Take the cells obtained from the primary generation to the 10th passage in Example 1, the cells obtained from the primary generation to the 8th passage in Example 1 two, and the cells of each generation obtained from the primary generation to the 9th passage in Example 1 3, respectively. The following identification experiments.
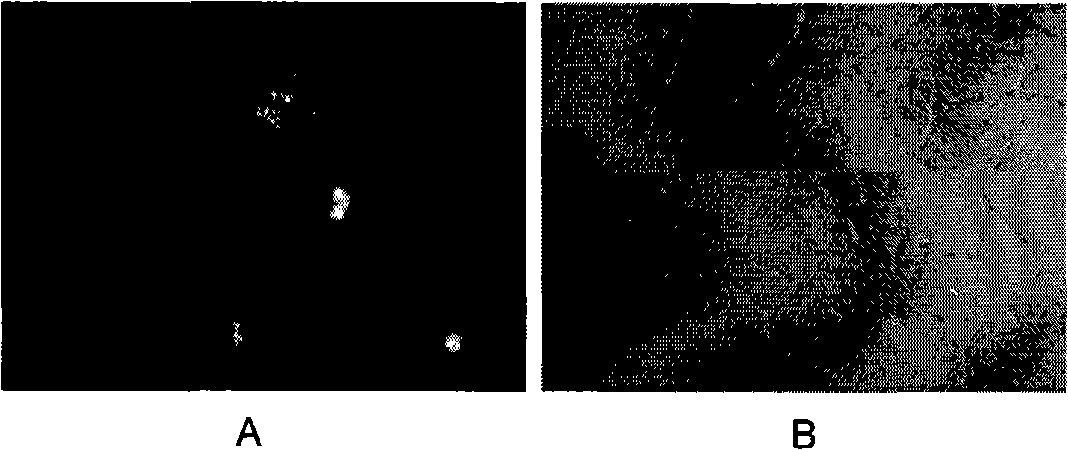
[0054] 1. Observation of cell morphology
[0055] During the growth process of each endothelial cell, several clones were formed in the 24-well plate, and the cells were in the shape of a spindle. When the cells covered the 24-well plate, the cells became thicker and shorter, and were in the shape of pebbles, such as obtained in Example 1-1. The morphology of the primary cells is as figure 1 as shown ( figure 1 A is fusiform, figure 1 B is pebble-like). It shows that, from the point of view of morphology, the cells obtained in the present invention conform to the morphological cha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com