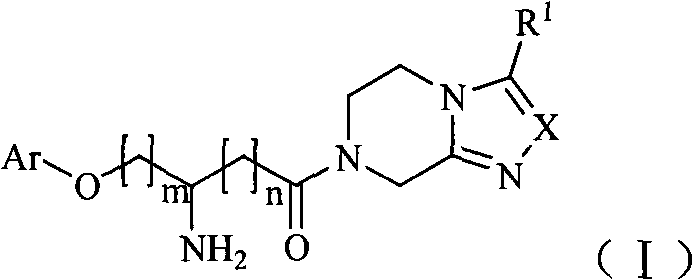
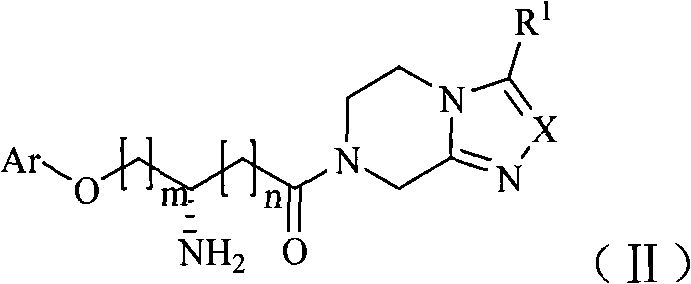
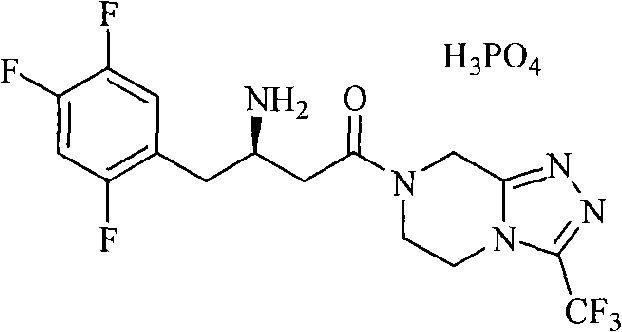
Dipeptidase inhibitor compound
A technology of compounds and compositions, applied in the field of medicine, can solve the problems of limited varieties of medicines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148] Example 1 7-[(R)-2-(tert-butoxycarbonylamino)-3-hydroxy-propionyl]-3-(trifluoromethyl)-5,6,7,8-tetrahydro-[1 ,2,4] Preparation of Triazolo[4,3-a]pyrazine
[0149]Throw 20.5g (100mmol) of (R)-2-(tert-butoxycarbonylamino)-3-hydroxy-propionic acid at room temperature, 13.5g (100mmol) HOBT (1-hydroxybenzotriazole), DMF100ml, Then 40g (150mmol) of DCC (dicyclohexylcarboimide) was added, and the reaction was stirred at room temperature for 2h. Remove the solid generated by the reaction by filtration, continue to add the filtrate to the reaction flask, add 19.2g (100mmol) 3-(trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazole And[4,3-a]pyrazine (see J.Med.Chem.2005, 48, 141-151 for its preparation method), raise the temperature and stir for 6h, after the reaction is complete, add 500ml of water to dilute, and precipitate a solid, and use methanol and dichloro The methane mixture was purified to obtain 30.4 g of the product, yield: 80.2%.
Embodiment 2
[0150] Example 2 7-[(R)-3-(tert-butoxycarbonylamino)-4-hydroxy-butyryl]-3-(trifluoromethyl)-5,6,7,8-tetrahydro-[1 ,2,4] Preparation of Triazolo[4,3-a]pyrazine
[0151] Referring to the preparation method of Example 1, throw (R)-3-(tert-butoxycarbonylamino)-4-hydroxy-butyric acid 21.9g (100mmol), 3-(trifluoromethyl)-5,6,7, 19.2 g (100 mmol) of 8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine. 7-[(R)-3-(tert-butoxycarbonylamino)-4-hydroxy-butyryl]-3-(trifluoromethyl)-5,6,7,8-tetrahydro-[1,2 , 4] Triazolo[4,3-a]pyrazine 29.4g, yield: 74.8%.
Embodiment 3
[0152] Example 3 7-[(R)-2-(tert-butoxycarbonylamino)-3-(N-2,4,5-trifluorophenoxy)-propionyl]-3-(trifluoromethane base)-5,6,7,8-tetrahydro-[1,2,4]triazol[4,3-a]pyrazine
[0153] 27.3 g (72 mmol) of 7-[(R)-2-(tert-butoxycarbonylamino)-3-hydroxyl-propionyl]-3-(trifluoromethyl)-5,6,7,8-tetra Add hydrogen-[1,2,4]triazol[4,3-a]pyrazine into the dry reaction flask, then add 11.8g (80mmol) of 2,4,5-trifluorophenol, 18.9g (72mmol) Triphenylphosphine, 200ml tetrahydrofuran. Cool down to below 0° C. and then add 12.5 g (72 mmol) of diethyl azodicarboxylate in 30 ml of toluene. Insulated and stirred for 50 hours, after the reaction was completed, the solvent was recovered under reduced pressure, the residue was dissolved in ethyl acetate, washed once with 1N sodium hydroxide and saturated sodium chloride solution, and purified on a silica gel column (ethyl acetate / cyclohexane) to obtain Product 18.8g, yield: 51.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com