Use of SIP in preparing medicine for preventing mesenchymal stem cells apoptosis
A bone marrow mesenchymal and stem cell technology, applied in the field of biomedicine, can solve the problems of low survival rate and poor treatment effect, and achieve the effect of improving survival rate, good effect and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1. In vitro test of S1P inhibiting bone marrow mesenchymal stem cell apoptosis
[0038] Proceed as follows
[0039] (1), MSCs isolation and culture
[0040] Sprague-Dawley rats, male or female, were taken, anesthetized with ketamine hydrochloride (60-80mg / kg) intraperitoneally, then soaked in 75% alcohol for 3-5 minutes for disinfection; Culture medium (IMDM+15% FBS) (both IMDM and FBS were purchased from Gibcol Biological Company) washed the bone marrow cavity 10 times, sucked and blown the bone marrow repeatedly with a micropipette to form a dispersed single-cell suspension, inoculated it in a culture bottle, and placed it at 37 °C, 5% CO 2 The cells were cultured in an incubator; after 5 days, the cells could grow to more than 80% confluence, and passaged at a ratio of 1:3 (0.25% trypsin) (trypsin was purchased from Sigma-Aldrich); the first-generation cells that grew well were used for experiments.
[0041] (2), experimental grouping and processing
[00...
Embodiment 2
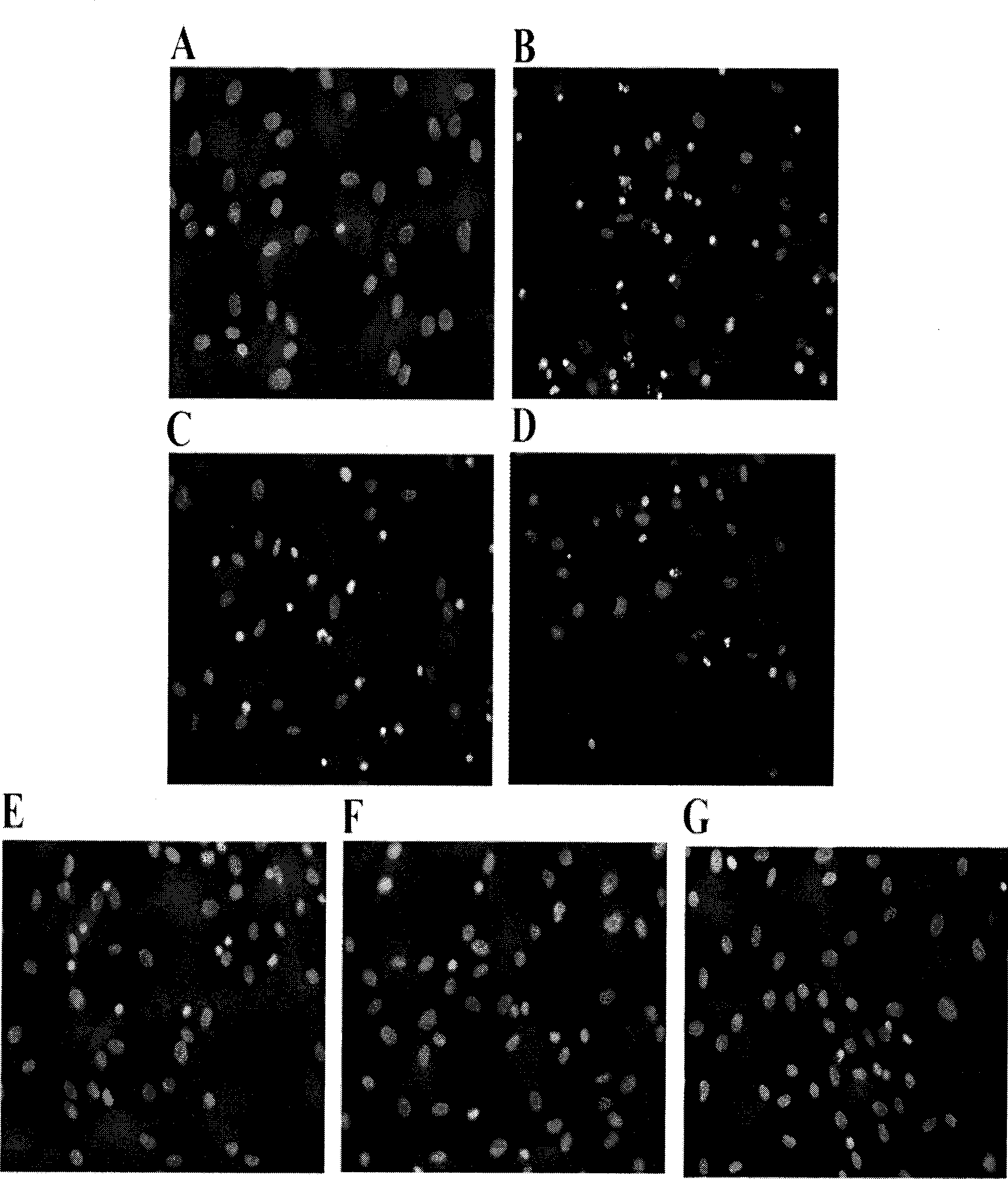
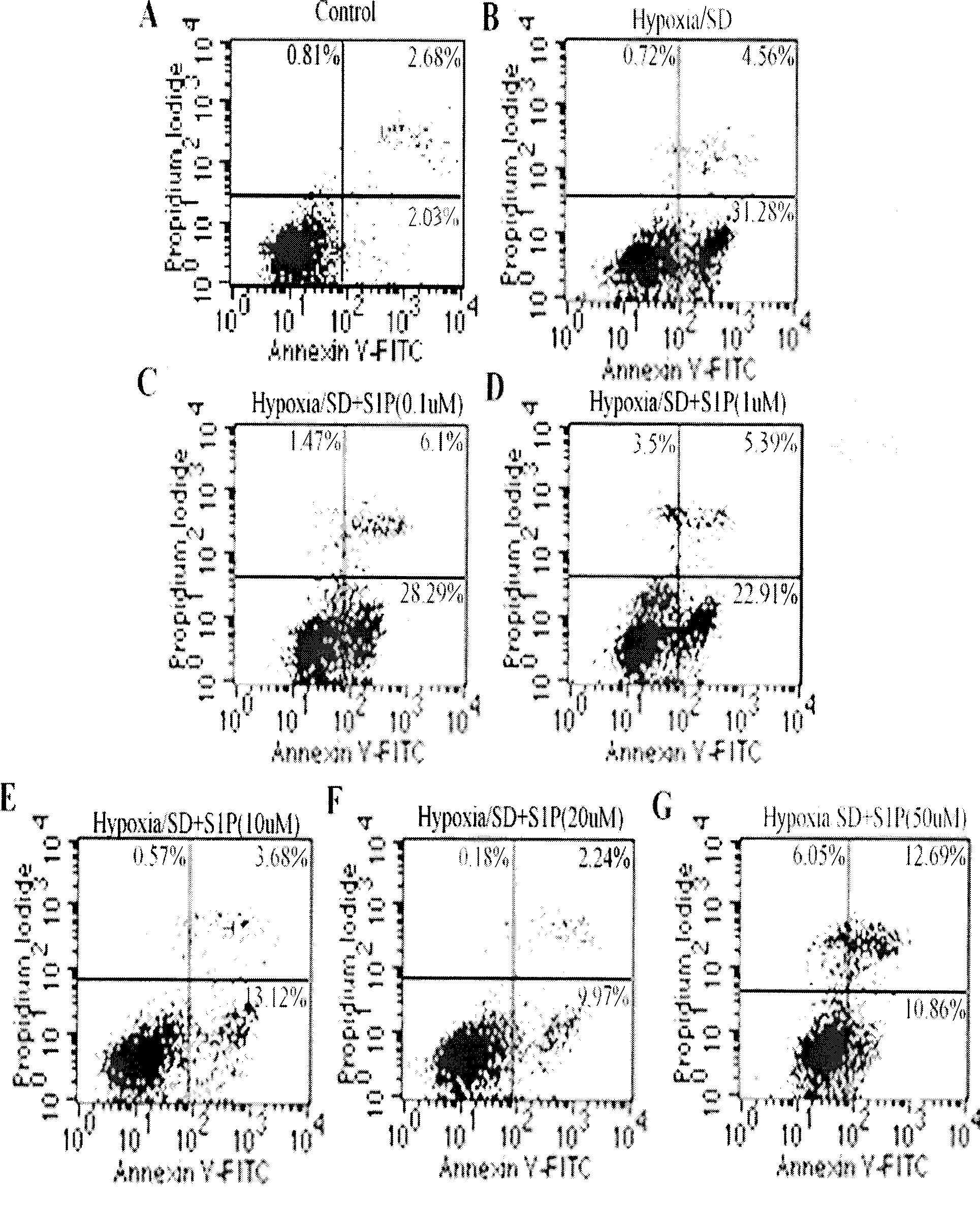
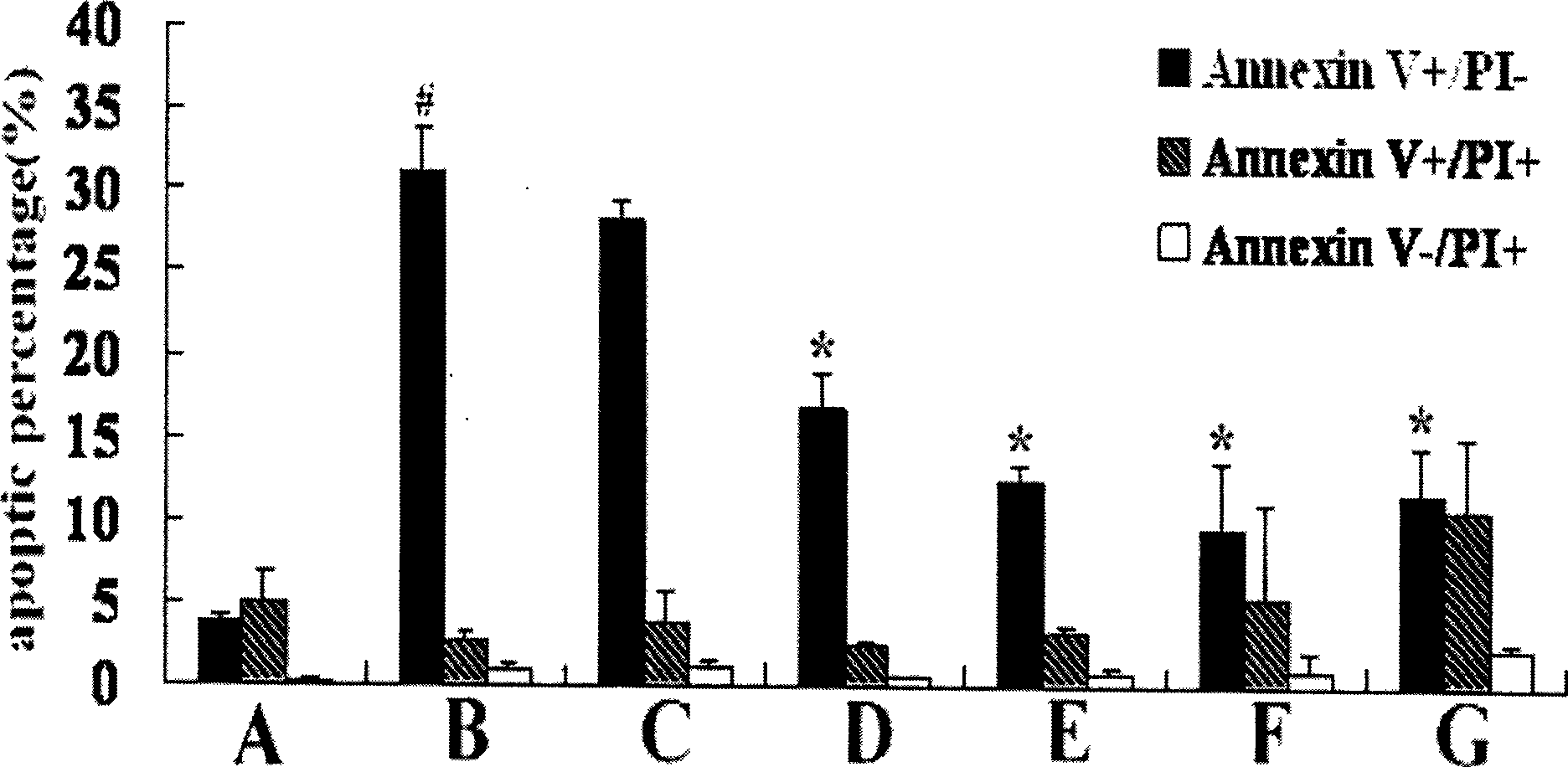
[0048] Example 2, Bone Marrow Mesenchymal Stem Cell Apoptosis Detection Test
[0049] The test method is as follows:
[0050] (1) Experimental grouping is the same as in Example 1.
[0051] (2) According to the instructions of the Annexin V / PI Apoptosis Detection Kit (Beijing Baosai Biotechnology Co., Ltd.), the cells of each group obtained in Example 1 were digested with trypsin, washed twice with cold PBS, and then washed with Annexin V / PI. V solution was incubated at room temperature for 30 minutes, then PI solution was added to incubate for 5 minutes, and then the apoptosis rate was detected by flow cytometry (Annexin-V / PI flow cytometry can distinguish early apoptosis from mid-late apoptosis of cells. Early apoptosis The PS on the cell membrane is everted to the outer layer of the cell membrane, showing Annexin-V positive, but the cell membrane is intact, and PI is negative, where Annexin V+ / PI- represents early cell apoptosis).
[0052] The results of the normal contro...
Embodiment 3
[0053] Embodiment 3, the test of the influence of S1P on caspase-3 activity
[0054] (1) Test treatment:
[0055] (a) Normal control group: normal culture with complete medium (IMDM+15% FBS); (b) apoptosis model group: treated with serum-free (IMDM) and hypoxia (placed in a closed hypoxia tank, built-in consumption Oxygen), 37°C, 5% CO 2 Incubate for 6 hours; (c) S1P (purchased from Sigma, USA) treatment group: MSCs were pretreated with 10uM S1P for 1 hour, and then incubated for 6 hours under hypoxic and serum-free conditions; (d) S1P (10μM) + apoptosis Model + LY294002 (25uM) group (LY294002 was purchased from cellsignal); (e) S1P (10 μM) + apoptosis model group + U0126 (20uM) group (U0126 was purchased from cellsignal).
[0056] (2) Test method: After 6 hours of hypoxia without serum, the cells of each group were digested with trypsin (0.25%), the cells were collected, and the cell protein was extracted, and the protein concentration was measured by the Coomassie brillian...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com