Medicinal composition for haemofiltration or hemodialysis
A technology of blood filtration and medicine, which is applied in the direction of drug combination, active ingredients of anhydride/acid/halide, active ingredient of alkali/alkaline earth metal chloride, etc. It can solve problems such as unstable ion concentration control and cumbersome preparation procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0018] 1. Two compartments or packs
[0019] Combination example 1:
[0020] Part A: Sodium lactate (sodium citrate, sodium malate, sodium tartrate, or sodium succinate), sodium chloride, magnesium chloride, and calcium chloride.
[0021] Part B: Sodium bicarbonate.
[0022] Combination example 2:
[0023] Part A: Sodium Chloride, Magnesium Chloride and Calcium Chloride.
[0024] Part B: Sodium lactate (sodium citrate, sodium malate, sodium tartrate, or sodium succinate) and sodium bicarbonate.
[0025] Combination example 3:
[0026]Part A: Magnesium Chloride and Calcium Chloride.
[0027] Part B: Sodium chloride, sodium lactate (sodium citrate, sodium malate, sodium tartrate, or sodium succinate) and sodium bicarbonate.
[0028] Combination example 4
[0029] Part A: Calcium Chloride.
[0030] Part B: Magnesium chloride, sodium chloride, sodium lactate (sodium citrate, sodium malate, sodium tartrate, or sodium succinate) and sodium bicarbonate.
[0031] 2. Three com...
Embodiment 1
[0053] Embodiment 1 Preparation of hemofiltration or dialysis solution of the present invention
[0054] The components used in the hemofiltration or dialysis fluid of the present invention are all in compliance with GMP regulations, and are prepared by blending the components with injection water. After screening and filtering steps known in the art, they are respectively filled into appropriate containers and sterilized. The formula of hemofiltration or dialysate of the present invention is as follows:
[0055] Recipe Example 1
[0056] Ingredient Content (mg / ml)
[0057] Sodium Lactate 0.90
[0058] Sodium chloride 5.90
[0059] Magnesium chloride·6H 2 O 0.20
[0060] Calcium chloride·2H 2 O 0.18
[0061] Sodium bicarbonate 2.69
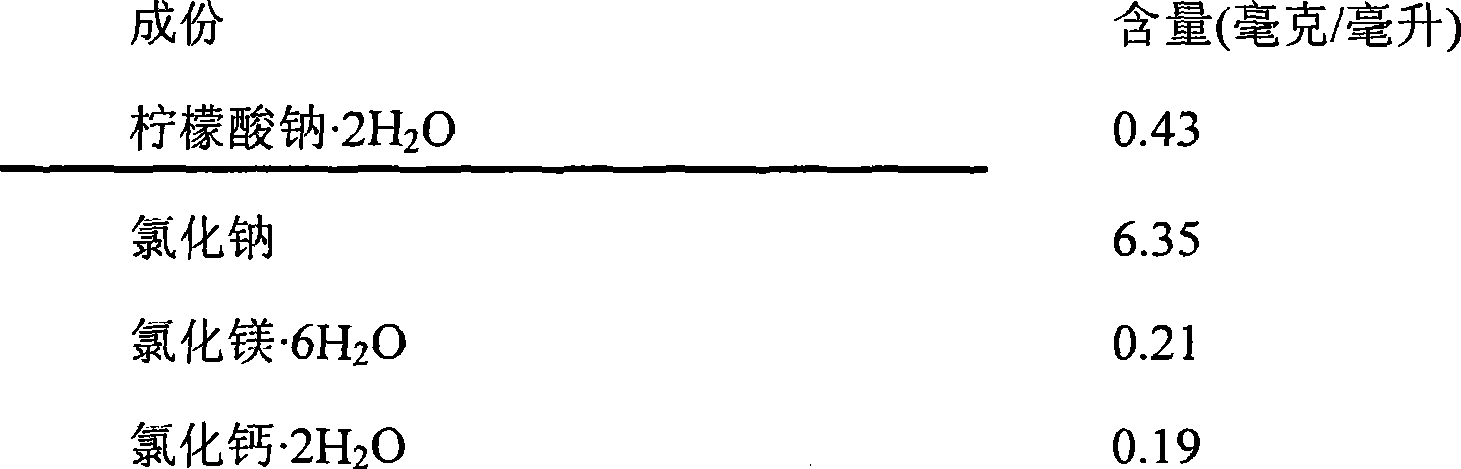
[0062] Recipe Example 2
[0063]
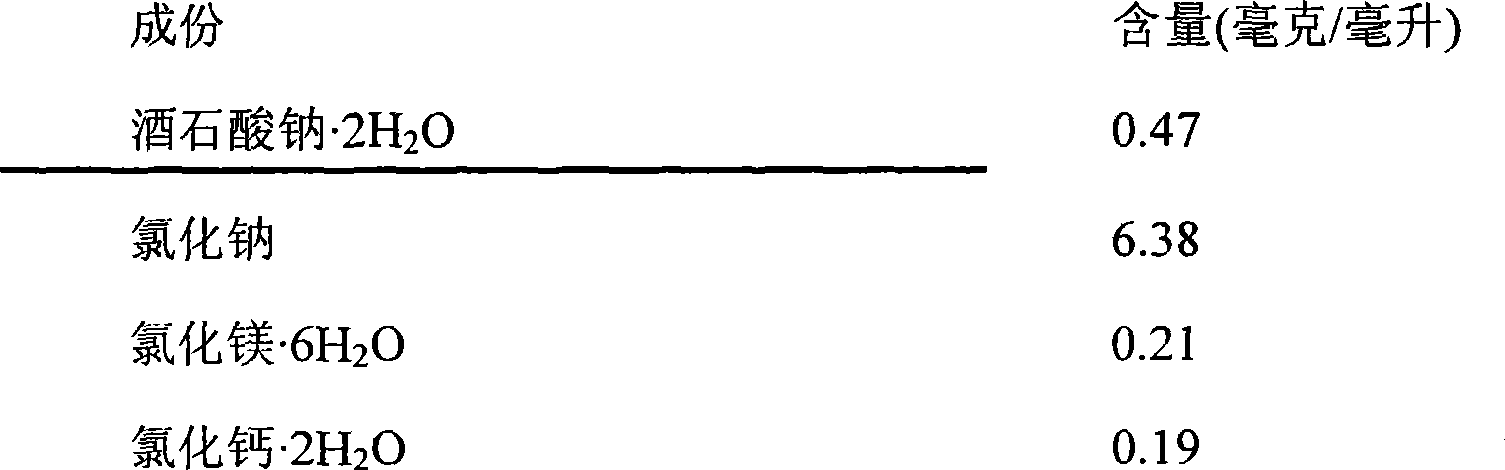
[0064] Recipe Example 3
[0065]
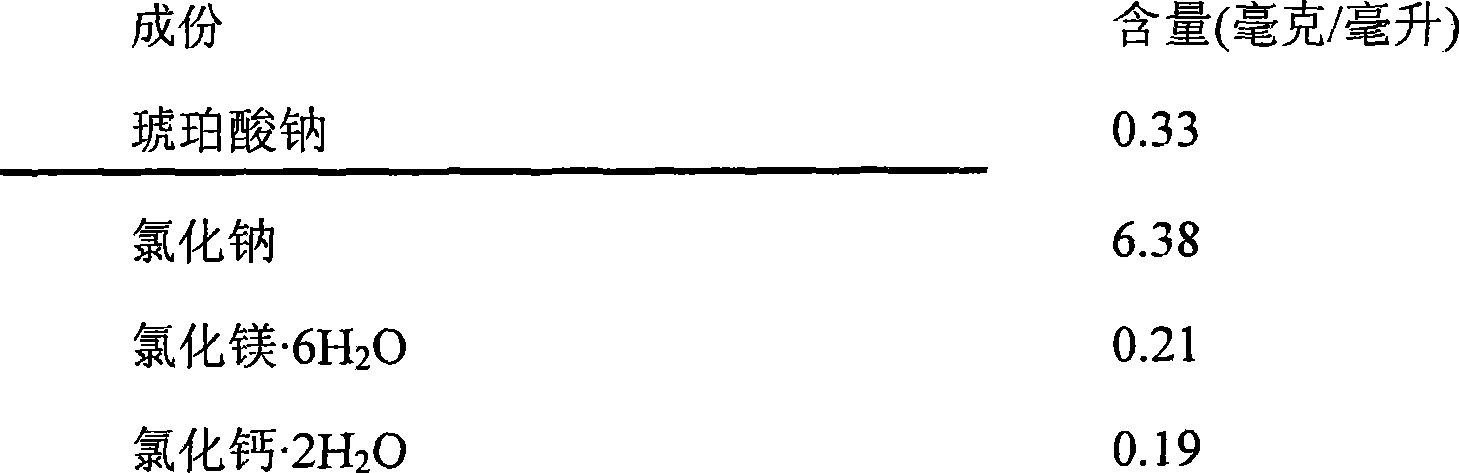
[0066] Recipe Example 4
[0067]
[0068] Recipe Example 5
[0069]
Embodiment 2
[0070] Embodiment 2 Preparation of medical packaging containing hemofiltration or dialysis fluid of the present invention
[0071] The ingredients in the following replenishment solutions A and B were blended with water for injection to form the supplement solutions A and B respectively. After screening and filtering steps known in the art, they are respectively filled into different compartments or packages of a medical container. The container is sterilized and forms the hemofiltration / dialysate product of the present invention. Add supplement B to supplement A at an appropriate time and mix uniformly to form the hemofiltration / dialysate of the present invention.
[0072] 1. Formulation Example 1
[0073] Divide the ingredients in the above formula example 1 into parts A and B and prepare them as follows:
[0074] 5L Supplement A and 200mL Supplement B
[0075]
[0076] after mixing Each ion / root concentration (mM) equivalent concentration (mEq) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com