Preparation method for hydroxyethyl group chitosan fiber
A technology of hydroxyethyl chitosan and chitosan fiber, which is applied in fiber processing, medical science, textiles and papermaking, etc., can solve the problems of increasing emissions or recycling costs, incomplete reaction, etc., and achieves degree of substitution control, replacement Easy to absorb and retain liquid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
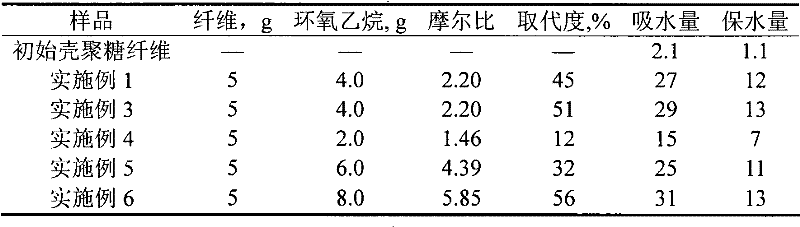
[0026] Immerse 5 g of chitosan fibers in 100 ml of a mixed solvent with a volume ratio of 35 / 65 ethanol / isopropanol, add 12.5 g of sodium hydroxide solution with a concentration of 25 wt %, and after alkalization at 10 ° C for 45 minutes, add 20 wt % of 20 g of an isopropanol solution of oxyethane (molar ratio 2.92), the system was sealed and heated to 40° C. to carry out hydroxyethylation modification. After two hours, the reaction was terminated, the fibers were taken out and dispersed in an 80 vol% ethanol aqueous solution, 10 vol% acetic acid aqueous solution was added dropwise to neutrality, and then washed with 80 vol% ethanol for three times, and finally dehydrated with absolute ethanol and dried in the air. The substitution degree of hydroxyethyl chitosan fiber is 45%.
Embodiment 2
[0028] Put 0.2 g of the fiber obtained in Example 1 into deionized water, take it out after 10 min, hang it for 30 s without droplets dripping, and weigh it. The water absorption of the fiber is 27 times its own weight. Then, the fiber after suction was placed in a centrifuge tube, and centrifuged at a speed of 1200 rpm for 15 min to separate the free liquid. The water retained by the fiber was 12 times its own weight. The water absorption and water retention of the initial chitosan fibers were 2.1 times and 1.1 times their own weight, respectively.
Embodiment 3
[0030] Immerse 5g of chitosan fibers in 100ml of a mixed solvent with a volume ratio of 35 / 65 ethanol / isopropanol, add 12.5g of sodium hydroxide solution with a concentration of 35%, and after alkalization at 10 ° C for 90 minutes, add 20wt% 20 g of isopropanol solution of oxyethane, the system is sealed and heated to 40°C for hydroxyethylation modification. After 4 hours, the reaction was terminated, the fibers were taken out and dispersed in 80 vol% ethanol aqueous solution, 10 vol% acetic acid aqueous solution was added dropwise to neutrality, washed three times with 80 vol% ethanol, and finally dehydrated with absolute ethanol and air-dried. The substitution degree of hydroxyethyl chitosan fibers was 51%, the liquid absorption was 29, and the water retention was 13.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com