Hepatitis b virus preS2 antigen chemiluminescence immune analysis determination reagent kit and preparing method thereof
A hepatitis B virus and chemiluminescence technology, applied in the field of immunoassay medicine, can solve the problems of radioactive waste pollution hazards, short validity period of radioimmunization reagents, low sensitivity of enzyme immunoassay reagents, etc. Fast, easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
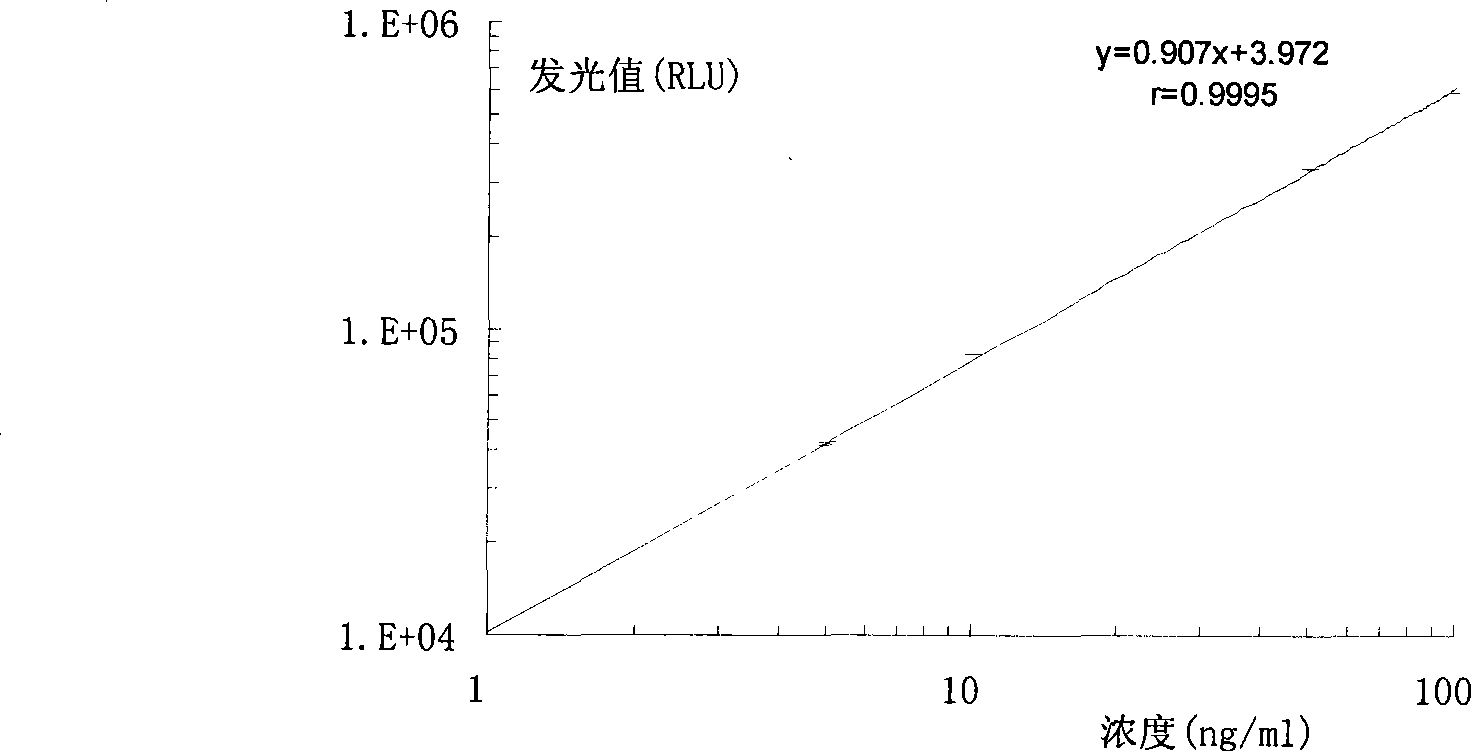
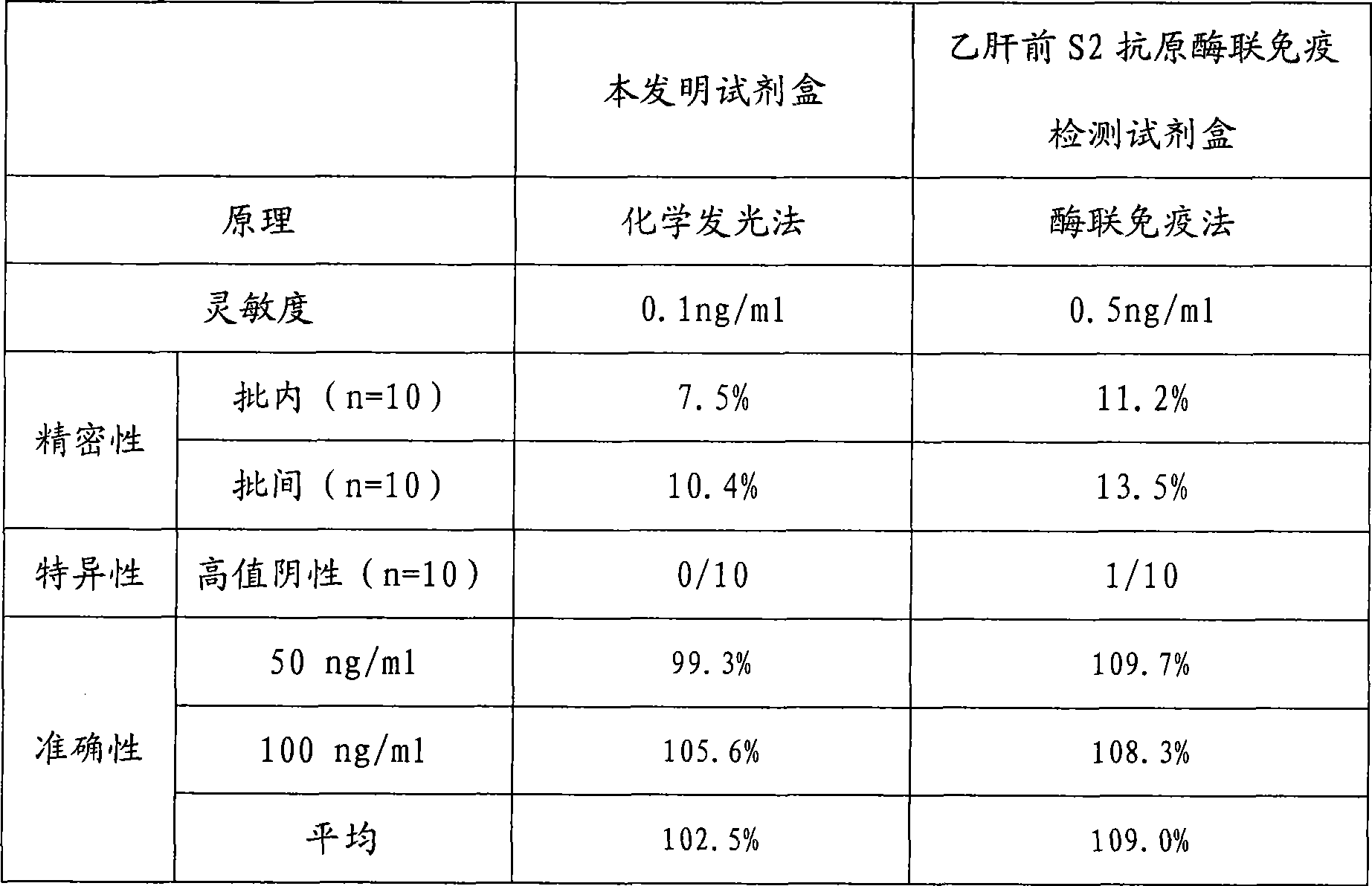
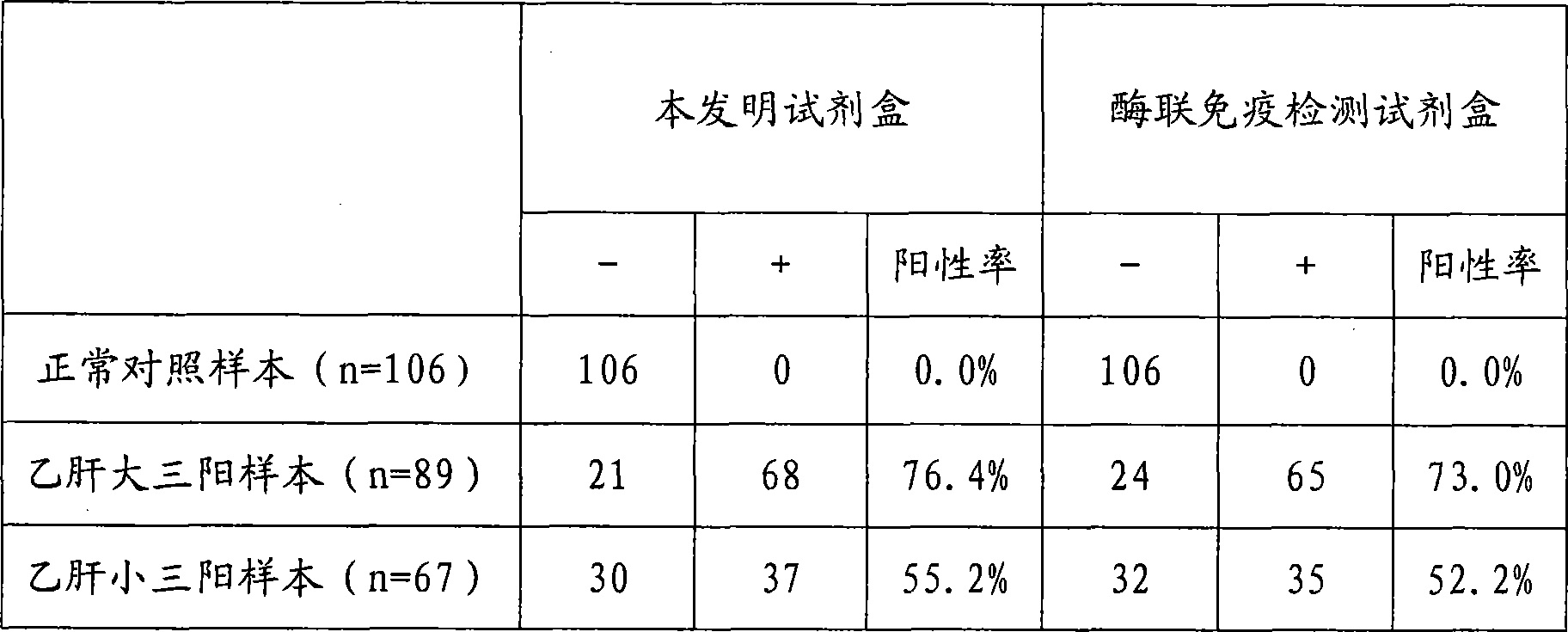
[0024] Example 1 Preparation of Hepatitis B Virus Pre-S2 Antigen Chemiluminescence Immunoassay Assay Kit of the present invention
[0025] During the research process of the present invention, the inventors firstly carried out screening tests and quality identification on the raw materials used, including the activities of labeled antibodies and biotinylated antibodies, the adsorption performance and precision of avidinized solid phase carriers, and chemiluminescence strength etc. Then the avidin solid-phase coating system was systematically studied, compared with different coating and blocking buffer systems, the most suitable coating buffer and blocking solution were selected, and the best coating was selected according to square matrix titration. By concentration. The inventor has repeatedly studied the biotin-conjugated antibody process and the alkaline phosphatase labeling process, and screened out a high-yield, low-cost, reliable, simple and easy-to-operate labeling met...
Embodiment 2
[0079] Example 2 Preparation of Hepatitis B Virus Pre-S2 Antigen Chemiluminescence Immunoassay Assay Kit of the present invention
[0080] Except that the plastic tube was used as the solid-phase carrier, the other methods were the same as in Example 1 to prepare the hepatitis B virus pre-S2 antigen chemiluminescent immunoassay assay kit.
Embodiment 3
[0081] Example 3 Preparation of Hepatitis B Virus Pre-S2 Antigen Chemiluminescence Immunoassay Assay Kit of the present invention
[0082] Except that the magnetic particles were used as the solid-phase carrier, and the avidin was coupled to the surface of the magnetic particles by the EDC method, the rest were prepared in the same manner as in Example 1 to prepare the hepatitis B virus pre-S2 antigen chemiluminescent immunoassay assay kit.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com