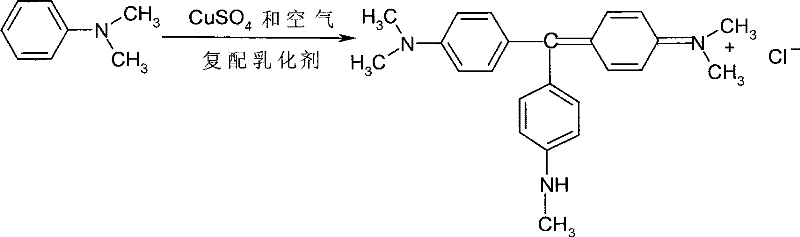
Synthetic technology of phenol-free alkali violet
A synthesis process and basic technology are applied in the catalytic oxidation synthesis of phenol-free basic violet dyes, and in the field of phenol-free basic violet synthesis technology, which can solve the problems of high cost, hard oxidizing material, low yield and the like, and reduce the recovery rate. The effect of copper cost, reduced emissions, simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Take 160ml of N,N-dimethylaniline aqueous solution (containing about 50g of N,N-dimethylaniline), take 5 grams of AEO-7 and 10 grams of chlorobenzene, 27g of salt, and 30g of copper sulfate crystals into the solution, Vigorously stir at 50-80°C and let in air. At the same time, add acetic acid dropwise to maintain acidity. React for 40-45 hours. The oxidation material is golden and cold and crisp at room temperature, which is the end of the reaction. Add 200ml of chlorobenzene and 100ml of liquid caustic soda and heat up to 100°C, stir for 3 hours, filter, let stand to separate layers, let go of the water layer, treat the organic layer with dilute hydrochloric acid, and pour off the organic phase to obtain 45g of dye. The dye strength is 100 after testing. points, the yield is more than 95%.
Embodiment 2
[0026] Example 2: Take 160ml of N,N-dimethylaniline aqueous solution (containing about 50g of N,N-dimethylaniline), take 4 grams of AEO-12 and 10 grams of chlorobenzene, 27g of salt, and 30g of copper sulfate crystals into the solution, Vigorously stir at 50-80°C and let in air. At the same time, add acetic acid dropwise to keep the acidity. React for 40-45 hours. The oxidation material is golden and crisp at room temperature, which is the end point of the reaction. The treatment method of the oxidation material is the same as in Example 1 to obtain 46g of dye. , the detected dye intensity is 100 points. The yield is above 95%.
Embodiment 3
[0027] Embodiment 3: get 160mlN, N-dimethylaniline aqueous solution (containing N, about N-dimethylaniline 50g), get 5 grams of AN1810 and 10 grams of chlorobenzene, 27g salt, 30g copper sulfate crystals and add solution, at 50 Vigorously stir at ~80°C and feed air. At the same time, add acetic acid dropwise to maintain acidity. React for 40 to 45 hours. The oxidation material is golden and cold and crisp at room temperature, which is the end of the reaction. The detection dye intensity is 100 points. The yield is above 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com