Intestinal alpha-glucosidase inhibitors and a process for the isolation and use thereof
A technology for glucosidase and inhibitory activity, which is applied in the field of α-glucosidase inhibitors and can solve problems such as application limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
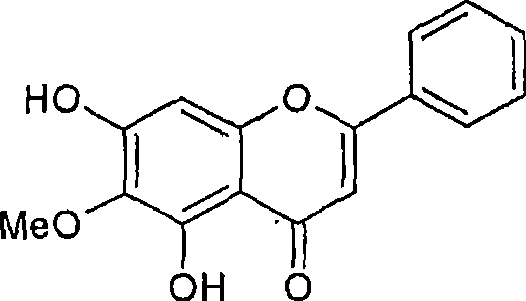
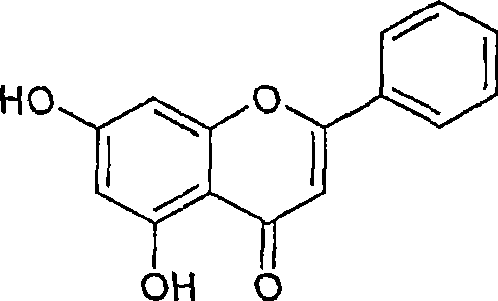
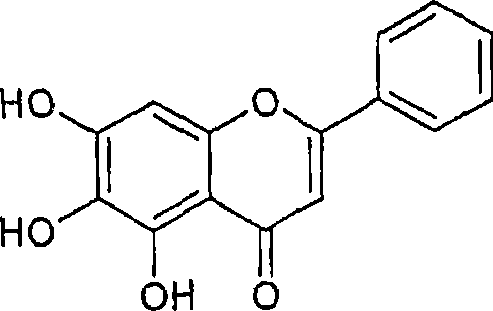
[0104] Experimental protocol: Method for isolating orogenin A, chrysin and baicalin.
[0105] Dry powdered stem bark (200 g) was first defatted with gasoline in a Soxhlet extractor. After filtering the hexane extract, a light yellow powdery solid was obtained. The solid (2 g) was chromatographed on a 3.5 cm diameter column packed with 60 cm high silica gel (60-120 mesh).
[0106] The column was continuously eluted with 1% methanol in chloroform to obtain orogenin A. The yield of orogenin A was about 1.2 grams.
[0107] The column was further eluted with 2% methanol in chloroform to give chrysin. The yield of chrysin was about 0.2 grams.
[0108] The column was further eluted with 3% methanol in chloroform to give baicalein. The yield of baicalein was about 0.5 grams.
[0109] In the hexane fractions such as oreolin A, chrysin, baicalin and unidentified compound, the ratio of the three active compounds to the unidentified compound was the following ratio 60:10:25:5.0.
[0...
Embodiment 2
[0120] Example 2: Determination of intestinal α-glucosidase inhibitory activity:
[0121] Rat intestinal acetone powder (sigma chemicals, USA) in normal saline at a ratio of 100:1 (weight / volume) was suitably sonicated. The supernatant obtained after centrifugation at 3000 rpm for 30 min at 25 °C was processed into a crude source of mammalian intestinal α-glucosidase. 10 microliter samples prepared from extracts or molecules (5 mg / ml in DMSO) were incubated with 50 microliters of crude enzyme in 100 microliters of 100 mM phosphate buffer (pH 6.8) for 5 minutes. Then 50 microliters of matrix (5 mM, p-nitrophenyl α-D-glucopyranoside prepared in buffer) was added. After 5 minutes of incubation, released p-nitrophenol was read spectrophotometrically at 405 nm. For control and blank readings, 10 microliters of DMSO was added at the sample and 50 microliters of buffer at the matrix. To compensate for interference from colored compounds, prepare a separate blank sample and evaluat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com