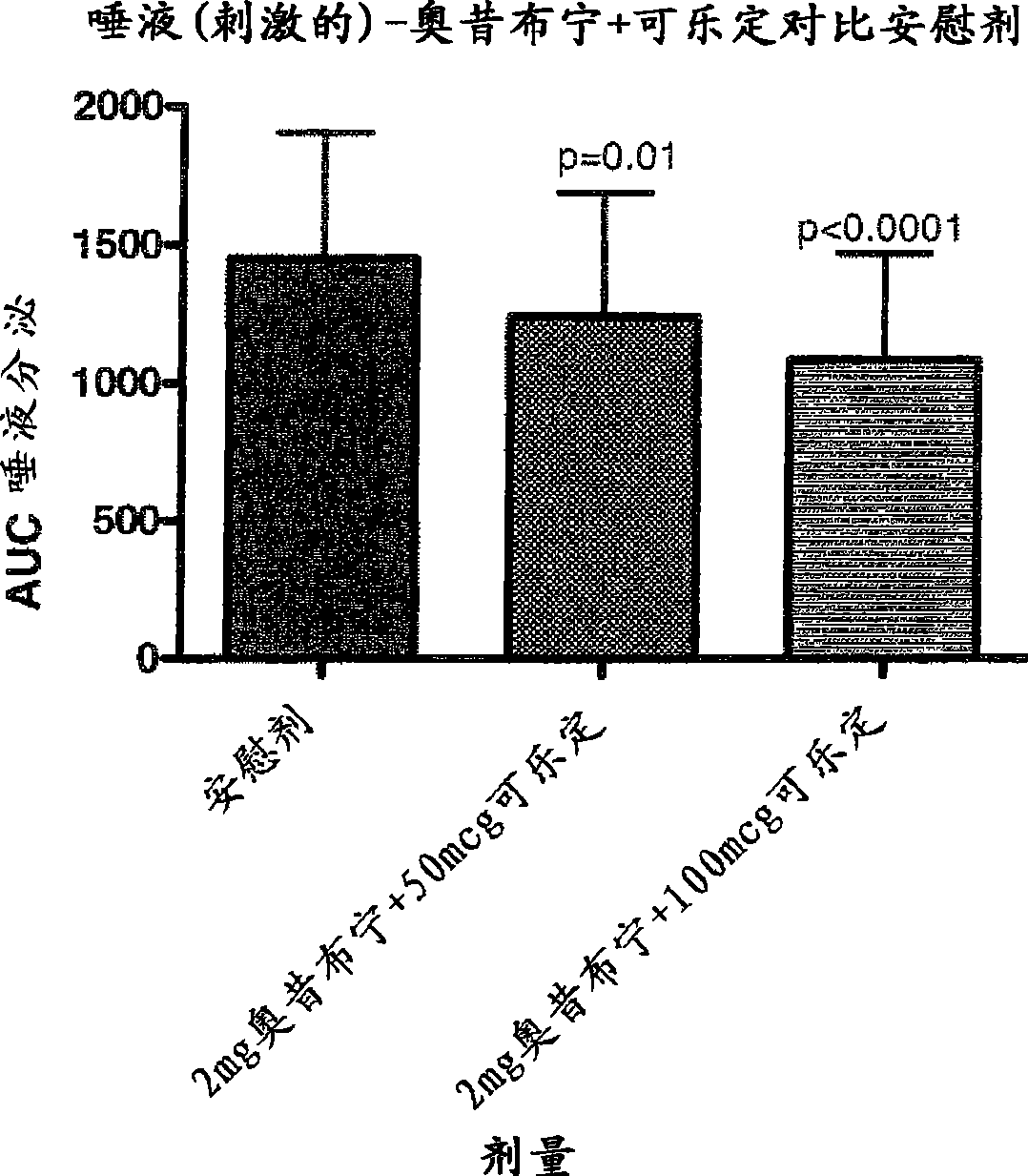
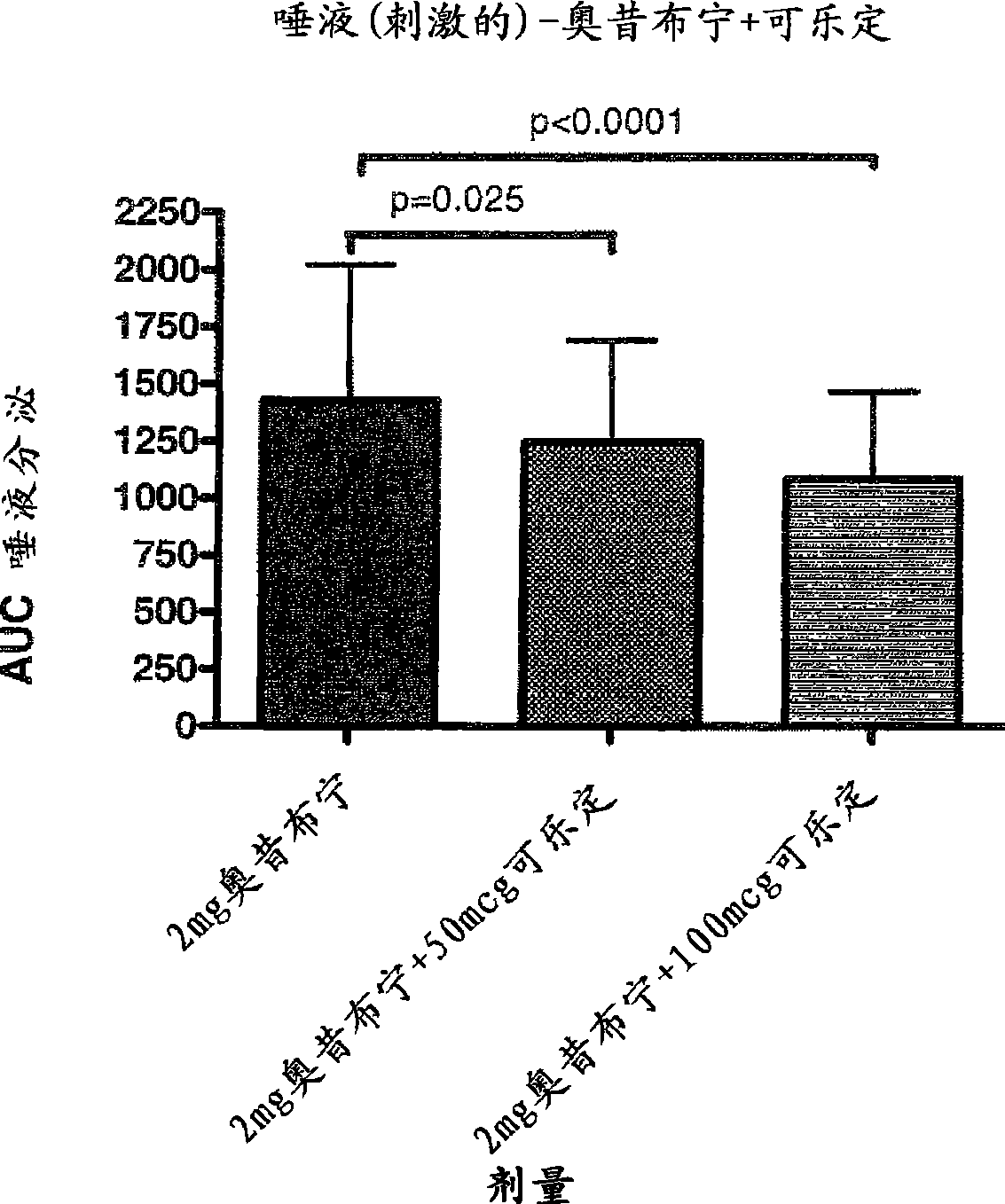
Combination of alpha-2 receptor agonist (clonidin) and an anti-muscarinic agent (oxybutynin) for the treatment of sialorrhoea
A receptor agonist and agonist technology, which is used in combination of α2 receptor agonist (clonidine) and antimuscarinic drug (oxybutynin) for the treatment of salivation, can solve problems such as unsatisfactory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0015] Preferred alpha2 adrenoceptor agonists for use in the present invention are clonidine, alaclonidine, brimonidine, rilmedinide, medetomidine, tizanidine, moxonidine and loxidine Fecidine. Preferred antimuscarinic drugs for use in the present invention are tolterodine, darfenacine, solifenacin, zafenacin, oxybutynin, tosbim, rivatropate, tiotropium Ammonium bromide and Ro-3202904 (PSD-506).
[0016] According to the invention, each active agent may be used in any suitable form, for example as a salt, hydrate or prodrug. In the case of chiral molecules, they may also be used in the form of racemates, non-racemic mixtures or essentially individual enantiomers.
[0017] In general, each active agent may be administered paralingually, sublingually or buccally in any suitable formulation. It is preferably formulated as a gum, spray, lozenge, lozenge or dispersible tablet.
[0018] The active agents can be formulated together in a single dosage form. Alternatively, they ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com