Target restraint alpha Nu beta3 positive cell proliferation cyclic peptides
A technology of positive cells and cyclic peptides, applied in the field of polypeptide drugs, to achieve the effect of inhibiting cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
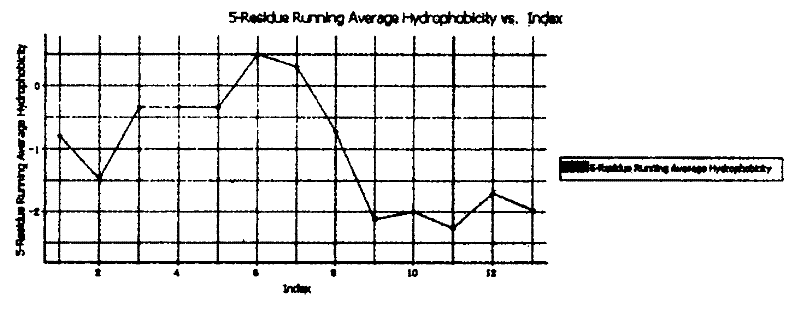
[0028] Embodiment 1, the physicochemical property analysis of polypeptide
[0029] The amino acid sequence of the polypeptide molecule of the present invention is input according to the format required by the ANTHPROT V5.0 software, and then various physical and chemical characteristic constants of the polypeptide molecule are calculated.
[0030] The results are shown in Table 1, figure 1
[0031] Table 1 Analysis results of polypeptide physical and chemical characteristic constants
[0032]
Embodiment 2
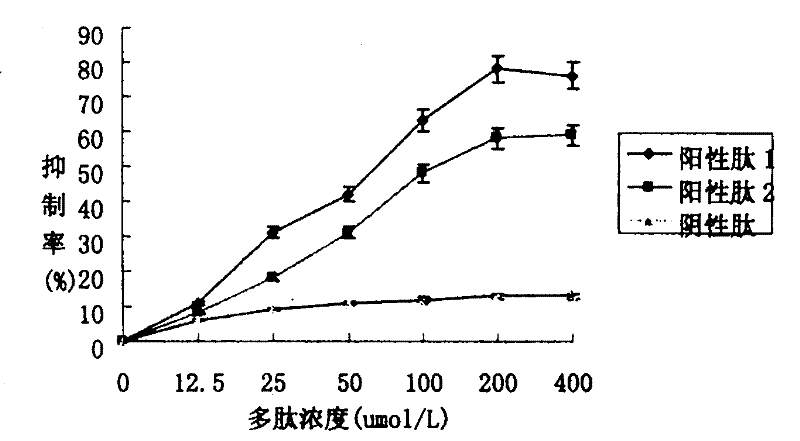
[0033] Example 2. Competitive inhibition experiments of polypeptides on the binding of nuclear factor-κB p65 subunits to DNA cis-acting elements
[0034] Polypeptide 1 is a linear peptide (RLRWRKC), and polypeptide 2 is a full-length cyclic peptide. The sequences were synthesized by Shanghai Huada Tianyuan Biotechnology Co., Ltd. with a purity of >95%. On a 96-well enzyme-linked plate coated with nuclear factor-κB cis-acting elements (purchased from Mercury TM Add 150 μl / well of transcription factor blocking solution to Transfactor p65 kits, and incubate at room temperature for 15 minutes; discard the transcription factor blocking solution, add 50 μl / well of detection samples diluted with transcription factor blocking solution, and the samples contain 10 ng / μl of p65 standard protein as Positive control, p65 standard protein containing 10ng / μl, add positive peptides at a series concentration at the same time, p65 standard protein containing 10ng / μl, add negative peptides at...
Embodiment 3
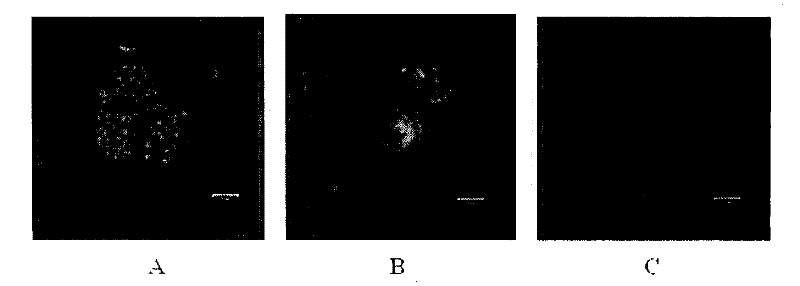
[0036] Example 3. Laser Confocal Detection of Cyclic Peptide Entry into αvβ3 Positive Cells
[0037] The cyclic peptides and markers in this experiment were synthesized by Shanghai Huada Tianyuan Biotechnology Co., Ltd. with a purity of >95%. In a cell culture system without lipofection transfection, the fluorescein (rhodamine)-labeled polypeptide (final concentration: 75 μM) was mixed with human umbilical vein endothelial cells, other αvβ3 positive cells (such as human breast cancer cells MDA-MB- 231) were co-cultured for 30 minutes, and co-cultured with αvβ3 negative cells (such as mouse macrophages) for 4 hours, and the results were observed under a confocal laser microscope.
[0038] The results showed that fluorescein-labeled polypeptides could be seen in human umbilical vein endothelial cells and human breast cancer cells MDA-MB-231, but even if the polypeptides were co-cultured with mouse macrophages for 4 hours, only a small amount of many peptides could be seen ente...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com