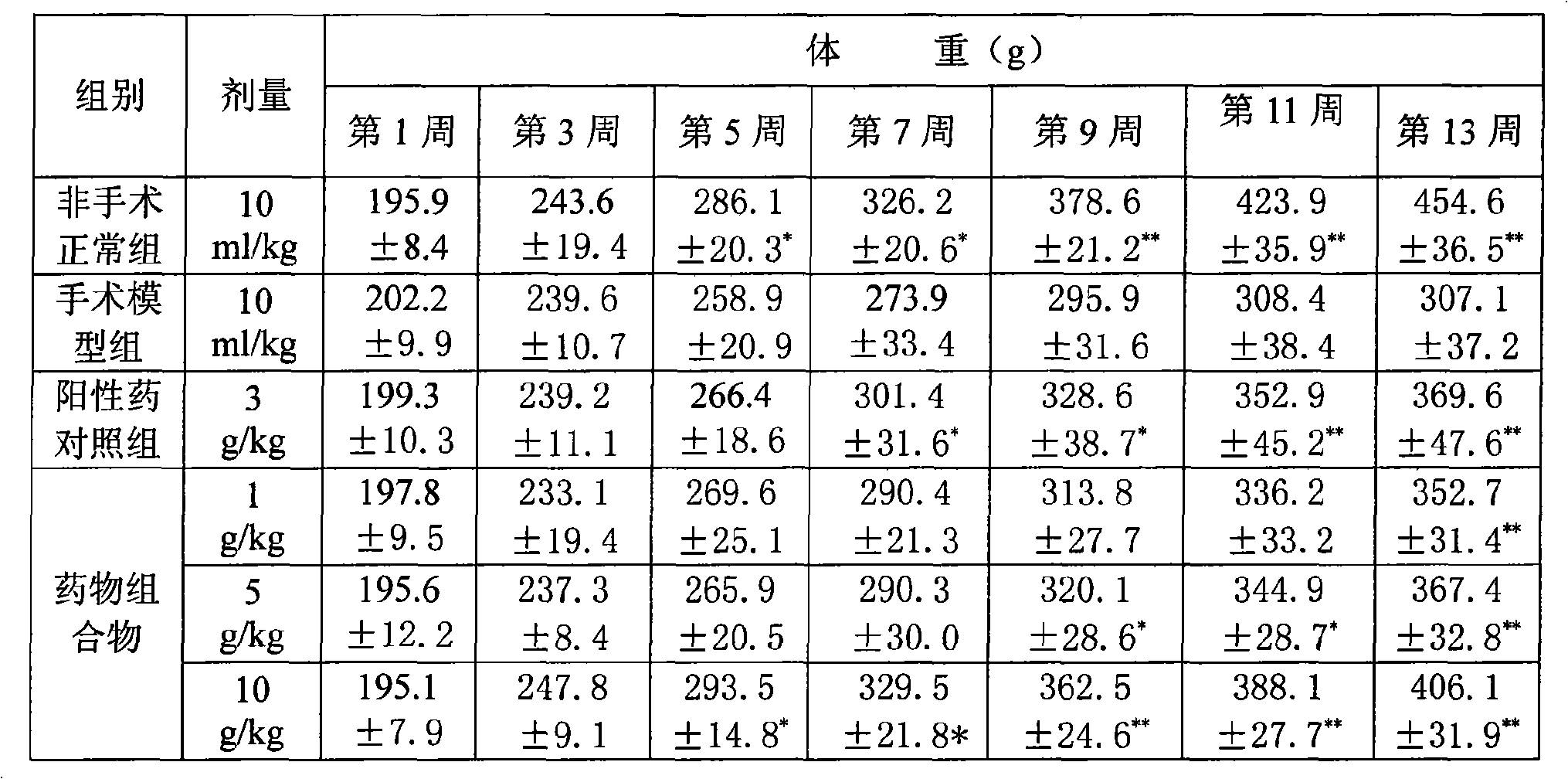
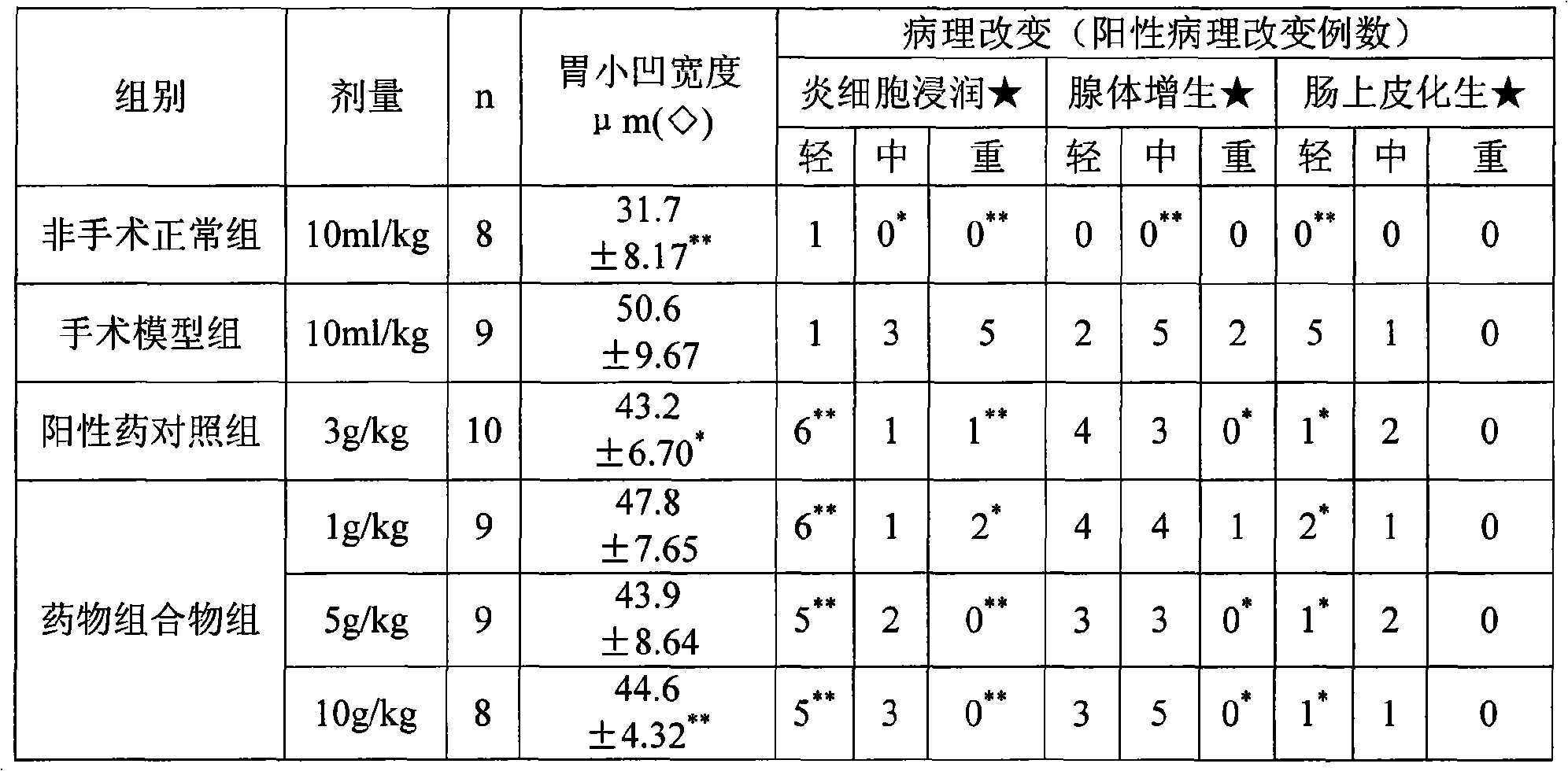
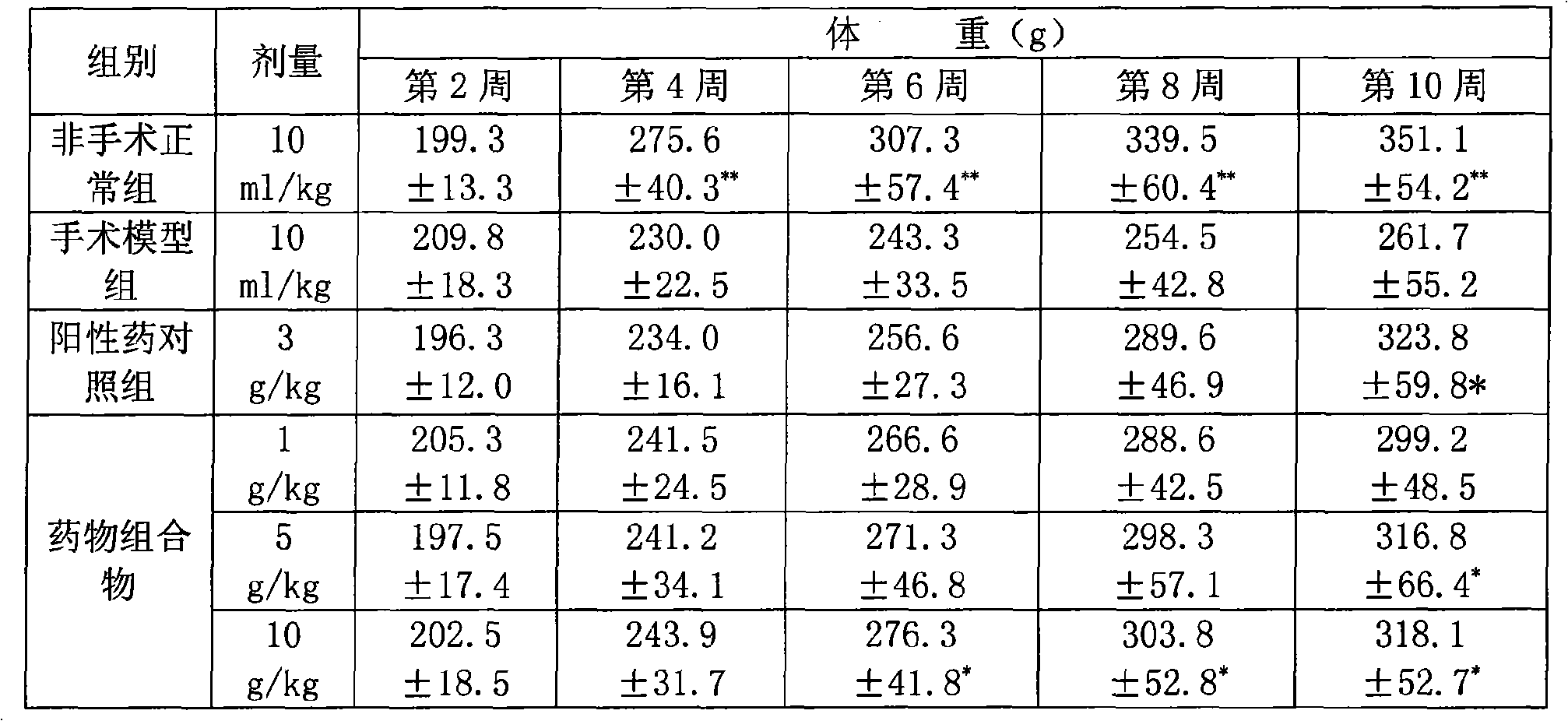
Pharmaceutical composition for treating chronic atrophic gastritis
A technology of atrophic gastritis and composition, applied in the field of pharmaceutical composition, which can solve the problems of insignificant curative effect, low patient compliance, large dosage of decoction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Take 6kg Sunburn Astragalus, 9kg Codonopsis Codonopsis, 3kg Tangerine Peel, 9kg Rhizoma Cyperi, 6kg Radix Paeoniae Alba, 6kg Chinese Yam, 2kg Black Plum, and 4kg Licorice, wash the above eight flavors, extract volatile oil from Tangerine Peel, and fry the remaining seven flavors in water Boil twice, 3 hours for the first time, and 2 hours for the second time (during the second decoction, add the dried tangerine peel residue after extracting the volatile oil), combine the decoction liquid, and filter. The filtrate was left to settle, and the supernatant was concentrated to a concentrated solution with a relative density of 1.05 to 1.10 (85°C thermal measurement), the concentrated solution was left to stand for more than 8 hours, the supernatant was filtered, and the filtrate was concentrated to a relative density of 1.14 to 1.16 ( Determination after diluting 2 times) clear ointment. Take 1 part of clear paste, add 1.75 parts of sucrose powder, 1.75 parts of d...
Embodiment 2
[0027] Example 2: Take 8 kg of Astragalus root, 6 kg of Codonopsis pilosula, 4 kg of tangerine peel, 6 kg of Cyperus cyperi, 12 kg of white peony root, 12 kg of Chinese yam, 5 kg of black plum, and 6 kg of licorice. Add water and decoct twice, the first time for 3 hours, and the second time for 2 hours (during the second decoction, add the residue of tangerine peel after extracting the volatile oil), combine the decoction liquid, and filter. The filtrate was left to settle, and the supernatant was concentrated to a relative density of 1.08-1.12 (by heat measurement at 85°C), and dextrin was added to obtain a concentrated solution with a relative density of 1.14-1.18 (by heat measurement at 85°C). The concentrated solution is spray-dried to make granules, then sprayed into tangerine peel volatile oil, and mixed evenly to obtain granules.
Embodiment 3
[0028] Example 3: Take 6 kg of Astragalus membranaceus, 5 kg of Codonopsis pilosula, 5 kg of tangerine peel, 6 kg of Cyperus cyperi, 6 kg of white peony, 6 kg of Chinese yam, 2 kg of black plum, and 4 kg of licorice, wash the above eight medicinal materials, extract volatile oil from the tangerine peel, and the remaining seven flavors Add water and decoct twice, the first time for 2 hours, and the second time for 2 hours (during the second decoction, the residue of tangerine peel after extracting the volatile oil is added), combine the decoction liquid, and filter. The filtrate was left to settle, and the supernatant was concentrated to a concentrated solution with a relative density of 1.00 to 1.10 (85°C thermal measurement), added volatile oil of dried orange peel, 10ml of essence, appropriate amount of preservative, 8.5kg of sucrose, mixed well, and added distilled water to make the whole amount into 30L, filter, fill, sterilize, and get ready.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com