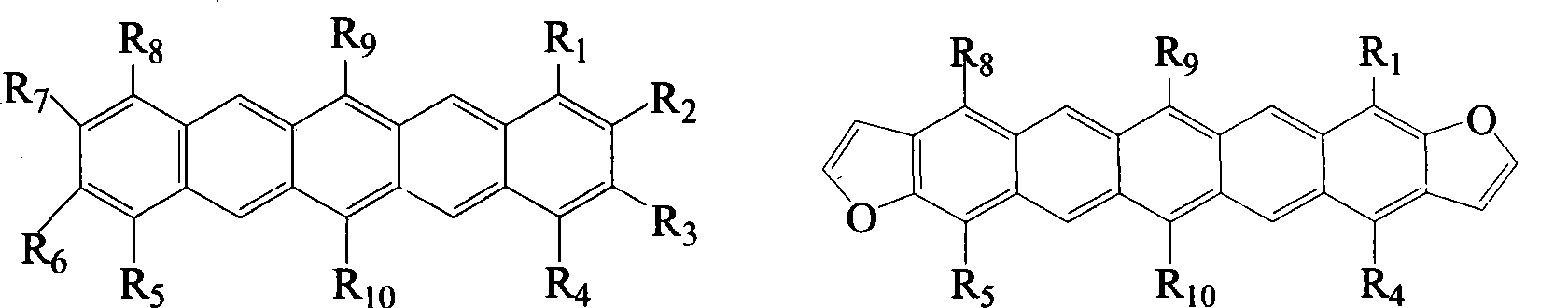
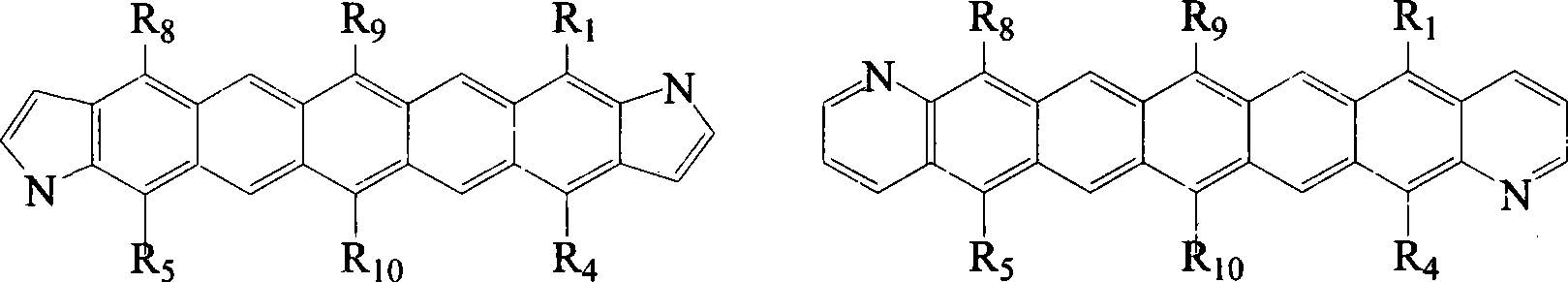
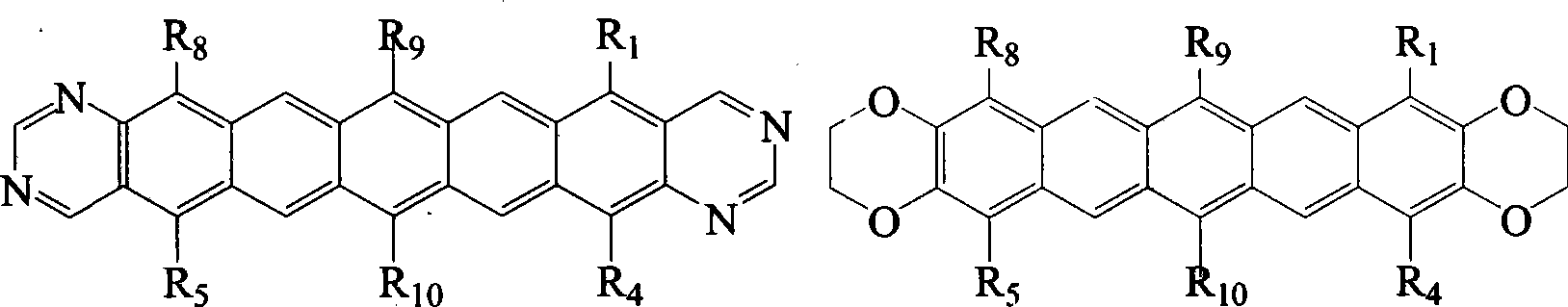
6,13-substituted pentacene and derivatives thereof, as well as preparation
A technology of pentacene and derivatives, applied in organic chemistry and other directions, can solve problems such as poor product performance, many reaction by-products, and complex reactions, and achieve the effects of good field effect mobility, simple steps, and good solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of 6,13-diphenylpentacene
[0037] Preparation of pentacenediquinone
[0038] Add 6.7g of o-phthalaldehyde, 2.8g of 1,4-cyclodihexanone and 70mL of ethanol into a 200mL three-necked flask to completely dissolve the solid. Then 35 mL of 10% KOH aqueous solution was added dropwise to the flask, and a precipitate formed. The above process requires constant stirring. After the KOH was added dropwise, the reaction was stirred for another 0.5 hour. The resulting mixture was suction-filtered, and the precipitate was washed with deionized water several times in small amounts until the filtrate was clear and colorless; then the precipitate was washed with absolute ethanol several times in a small amount until the filtrate was clear and colorless; Washing; Finally, after vacuum drying, pentacenediquinone is obtained.
[0039] Preparation of Grignard reagents
[0040] Add 0.6g of magnesium powder and a small grain of iodine into a dry 100mL three...
Embodiment 2
[0043] Example 2: Preparation of 2,3,9,10-tetramethyl-6,13-diphenylpentacene
[0044] Preparation of 2,3,9,10-tetramethylpentacenediquinone
[0045] 7.9 g of 3,4-dimethyl-phthalaldehyde, 2.8 g of 1,4-cyclodihexanone and 70 mL of ethanol were added into a 200 mL three-necked flask to completely dissolve the solid. 35 mL of 10% KOH aqueous solution was added dropwise to the flask. The above process requires constant stirring. After the dropwise addition of KOH, the reaction was stirred for another 1 h. The resulting mixture was suction-filtered, and the precipitate was washed with deionized water several times in small amounts until the filtrate was clear and colorless; then the precipitate was washed with absolute ethanol several times in a small amount until the filtrate was clear and colorless; washing; and finally vacuum drying to obtain 2,3,9,10-tetramethylpentacenediquinone.
[0046] Preparation of Grignard reagents
Embodiment 3
[0050] Embodiment 3: the preparation of 6,13-two (1-naphthyl) pentacene
[0051] Preparation of pentacenediquinone
[0052] 6.7 g of o-phthalaldehyde, 2.8 g of 1,4-cyclodihexanone and 70 mL of ethanol were added into a 200 mL three-necked flask to completely dissolve the solid. Further, 35 mL of 15% NaOH aqueous solution was added dropwise to the flask. The above process requires constant stirring. After NaOH was added dropwise, the reaction was stirred for another 0.5 hour. The resulting mixture was suction-filtered, and the precipitate was washed with deionized water several times in small amounts until the filtrate was clear and colorless; then the precipitate was washed with absolute ethanol several times in a small amount until the filtrate was clear and colorless; Washing; finally vacuum drying to obtain pentacenediquinone.
[0053] Preparation of Grignard reagents
[0054] Add 0.2g of magnesium powder and a small grain of iodine into a dry 100mL three-necked flask,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com