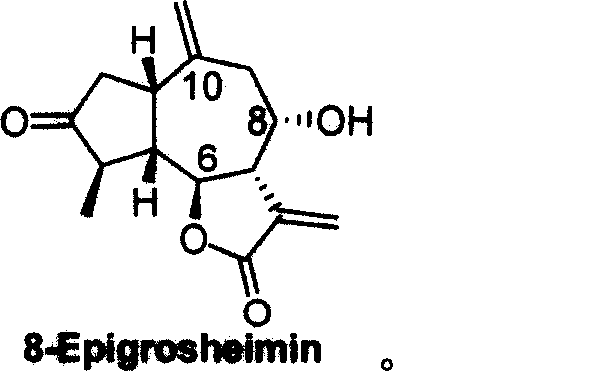
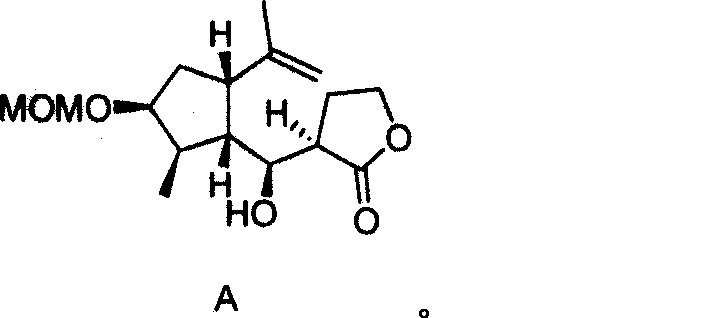
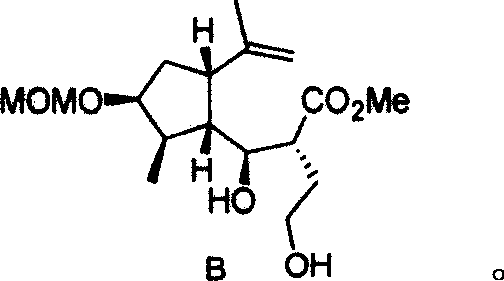
Anti-ameba protozoon disease compounds of 8-Epigroshiemin and synthesis thereof
A technology of amoeba and synthetic method, applied in organic chemistry, anti-infective drugs, drug combination, etc., can solve the problems of low content, no further report on activity, and difficult synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0025] The synthetic method of the anti-amoeba compound Epigrosheimin [(—)-8-Epigrosheimin provided by the invention comprises the steps:
[0026]
[0027] The specific synthesis steps are described in detail as follows:
[0028] 1. The synthesis of the raw material 2-methyl-3-methoxymethyl-5-isopropenyl-cyclopentanecarbaldehyde (5-Isopropenyl-3-methoxymethoxy-2-methyl-cyclopentanecarbaldehyde) used in the reaction is the method with reference to the literature Synthesized with a yield of 50%. (Lee E, Yoon C H. Stereoselective favorskii rearrangement of carvone chlorohydrin: Expedient synthesis of (+)-dihydrone petalactone and (+)-iridomyrmecin. JChem Soc, Chem Commun, 1994: 479-481).
[0029] 2. Synthesis of Intermediate 1:
[0030]2-Methyl-3-methoxymethyl-5-isopropenyl-cyclopentanecarbaldehyde (300mg, 1.4mmol) and trimethylsilyl enol of γ-butyrolactone at -78°C A solution of boron trifluoride (0.27 mL, 2.1 mmol) in dichloromethane (2.0 mL) was added dropwise to a solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com