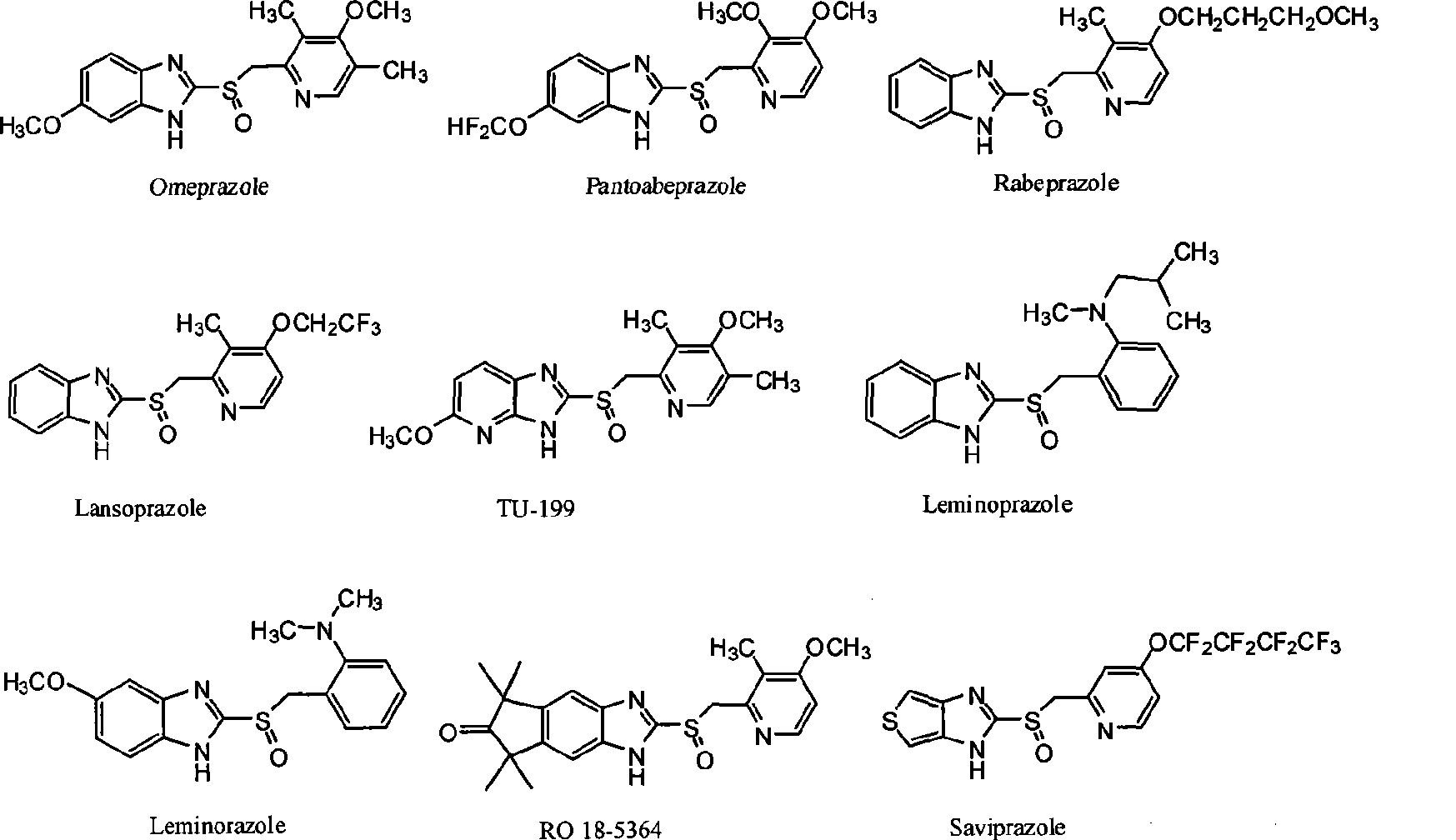
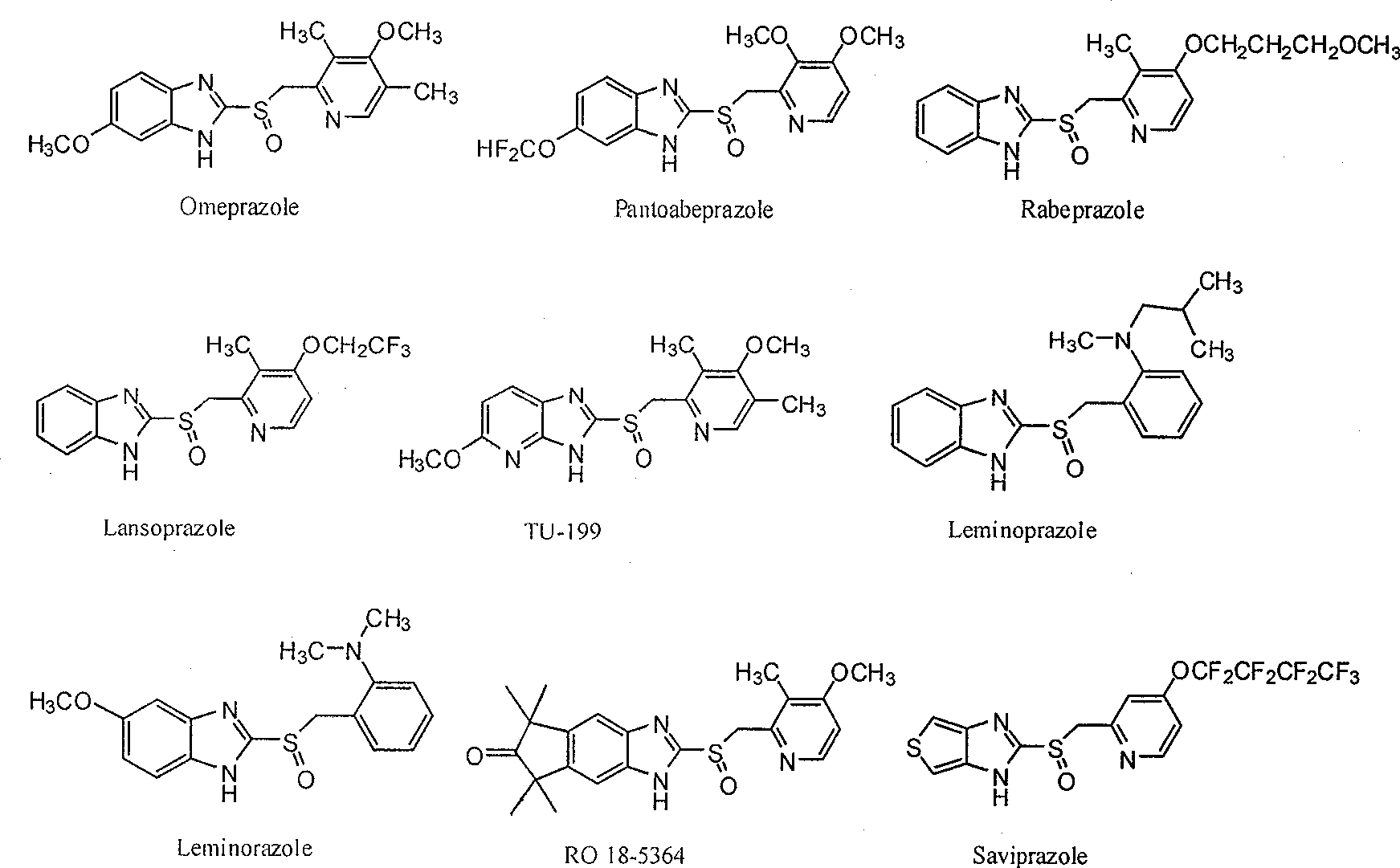
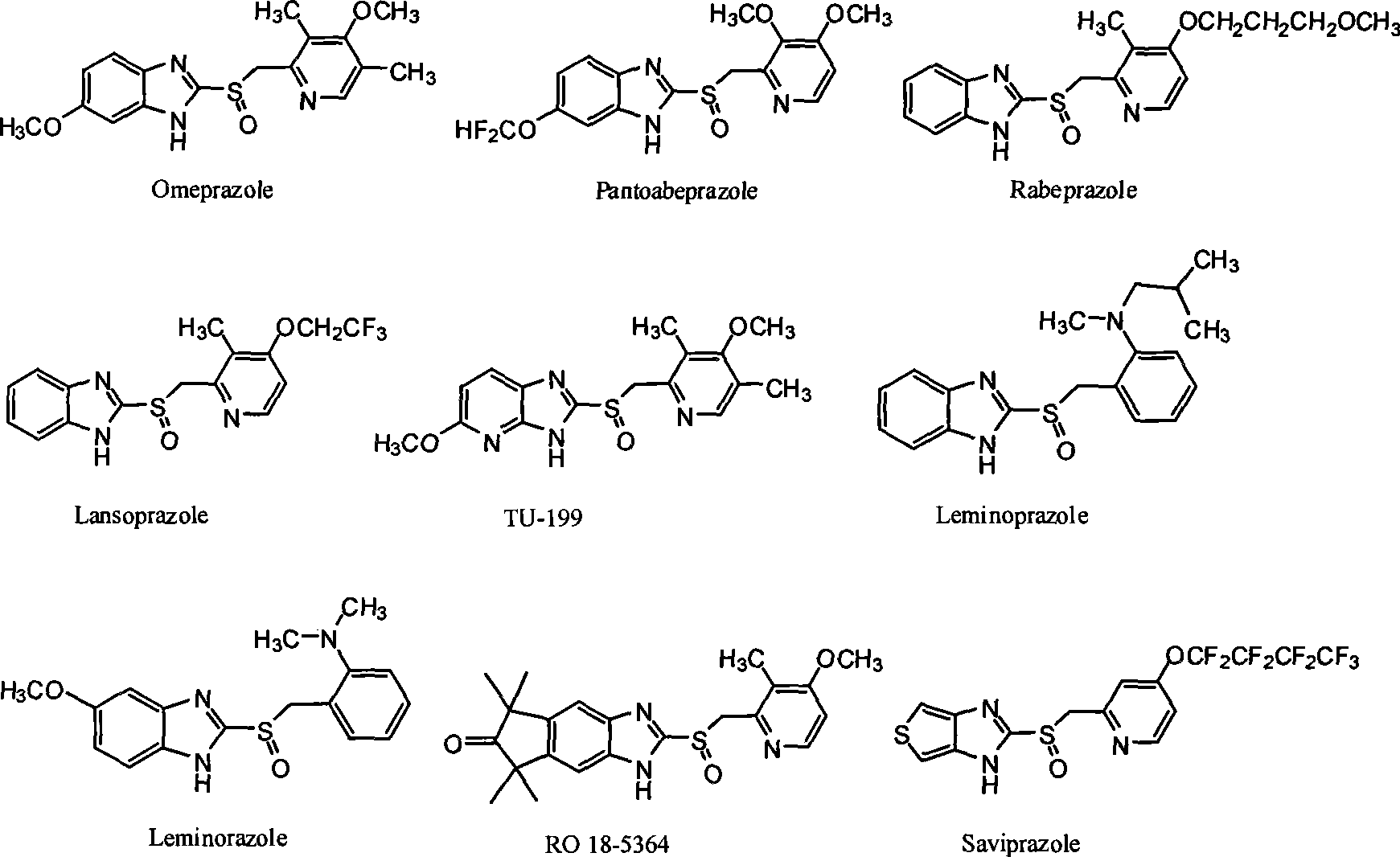
Novel method for producing chiral sulfoxide derivant
A derivative and chiral technology, applied in drug combination, organic chemistry, digestive system, etc., can solve the problems of peroxidation oxidation yield, purification difficulty, yield and ee value reduction, etc., to achieve mild reaction and simple preparation process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Synthesis of (-)5-difluoromethoxy-[[(3,4-dimethoxy-2-pyridyl)methyl]sulfinyl]-1H-benzimidazole, (S)-pantrop Larazole
[0021] 5-Difluoromethoxy-2-[(3,4-dimethoxy-2-pyridyl)methyl]thio-1H-benzimidazole (183.5g, 0.50mol) was suspended in 1000ml of toluene, Add D-diethyl tartrate (62.4g, 0.30mol), tetraisopropyl titanate (42.6g, 0.15mol), H 2 O (1.15ml, 0.064mol). Raise the temperature to 60-65°C, heat and stir for 1 hour to form a transparent solution, cool down to 0-5°C, add diisopropylethylamine (25.5ml), and slowly add dicumyl hydroperoxide dropwise within 3 hours ( DCHP, 294g, 47%, 0.71mol), insulation reaction for 20 hours, HPLC detection containing pantoprazole 97.7%, thioether 0.7%, add 500ml10% sodium hydroxide, stir for half an hour, layering, organic layer with 10% Extract with sodium hydroxide (250ml×3), discard the organic layer, combine the lye, add 750ml of methanol and 10g of activated carbon, stir for half an hour, filter, adjust the pH of the filtrate ...
Embodiment 2
[0023] Synthesis of (+)5-difluoromethoxy-[[(3,4-dimethoxy-2-pyridyl)methyl]sulfinyl]-1H-benzimidazole, (R)-pantrop Larazole
[0024] 5-difluoromethoxy-2-[(3,4-dimethoxy-2-pyridyl)methyl]thio-1H-benzimidazole (11.1g, 30.0mmol) was suspended in 60ml of toluene, Add L-diethyl tartrate (3.12ml, 18.2mmol), tetraisopropyl titanate (2.64ml, 9.0mmol), H 2 O (69 μl, 0.038 mmol). Raise the temperature to 60-65°C, heat and stir for 1 hour to form a transparent solution, cool down to 0-5°C, add diisopropylethylamine (1.53ml), and slowly add dicumyl hydroperoxide dropwise within 3 hours ( DCHP, 6.9g, 70%, 0.71mol), react at room temperature for 48 hours, add 60ml of 10% sodium hydroxide, stir for half an hour, separate layers, extract the organic layer with 10% sodium hydroxide (30ml×3), discard the organic layer , combined the lye, adjusted the pH to 7.5-8.0 with acetic acid, extracted with ethyl acetate, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pres...
Embodiment 3
[0026] (-) Synthesis of 2-[[(3,5-dimethyl-4-methoxyl-2-pyridyl)methyl]sulfinyl]-5-methoxyl-1H-benzimidazole, ( S)-Omeprazole
[0027] Suspension of 2-[[(3,5-dimethyl-4-methoxy-2-pyridyl)methyl]thio]-5-methoxy-1H-benzimidazole (1.63 g, 5 mmol) In 10ml of toluene, add D-diethyl tartrate (0.62g, 3.03mmol), tetraisopropyl titanate (0.43g, 1.5mmol), H 2 O (11.5 μl, 0.64 mmol). Raise the temperature to 60-65°C, heat and stir for 1 hour to form a transparent solution, cool down to 0-5°C, add diisopropylethylamine (0.26ml), slowly add dicumyl hydroperoxide (DCHP, 2.94g , 47%, 7.1mmol), insulation reaction overnight, HPLC detection containing 95.4% omeprazole, 0.3% thioether, add 5ml10% sodium hydroxide, stir for half an hour, separate layers, and extract the organic layer with 10% sodium hydroxide (10ml×3), the organic layer was discarded, the lye was combined, 1g of activated carbon was added, stirred for half an hour, filtered, the filtrate was adjusted to pH=7.5-8 with acetic ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com