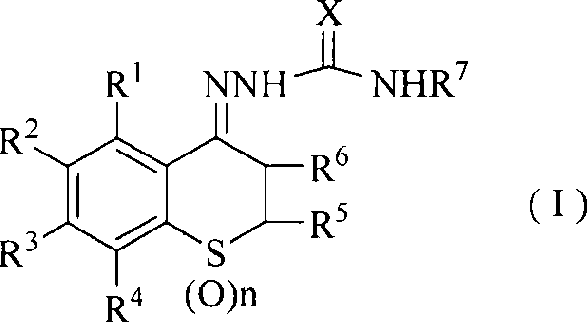
Antifungal agent-sulfur chromanone thiosemicarbazone compounds
A kind of technology of thiosemicarbazone and thiochromanone, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
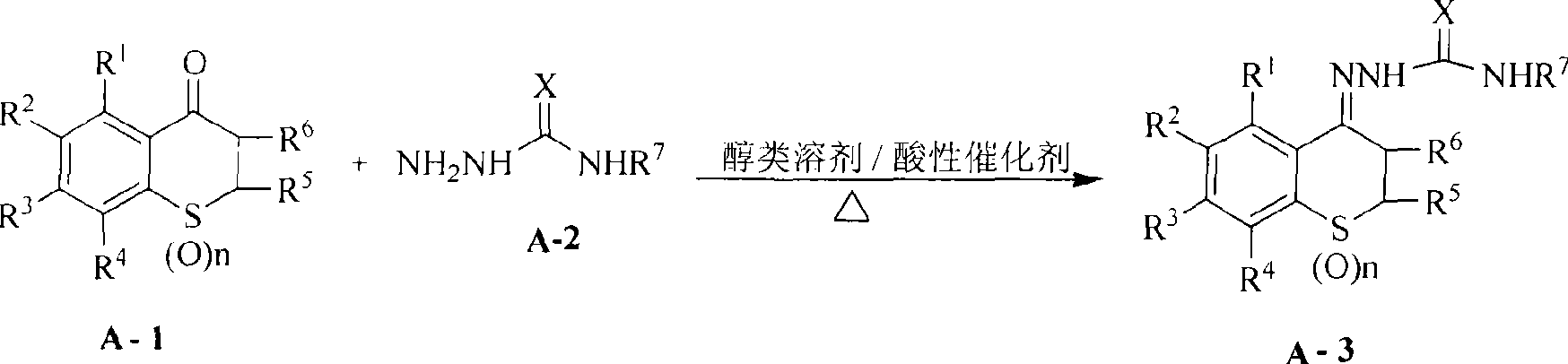
Method used
Image
Examples
Embodiment 1
[0081] 1-(6,8-dichloro-4-thiochromanone) semicarbazone
[0082] 23.3g (0.1mol) of 6,8-dichloro-4-thiochromanone, 75g (0.1mol) of semicarbazide, and 40.5g (0.05mol) of sodium acetate were dissolved in 100ml of ethanol, and the pH of the reaction solution was adjusted with hydrochloric acid= 3. Heat to reflux for 2 hours, cool to room temperature, precipitate solid, filter with suction, rinse with a small amount of ethanol, and dry to obtain a crude product. The product obtained by recrystallization with n-butanol as a solvent is a white solid, the yield is 24g, the yield is 83%, mp: 197~199°C, LC-MS (m / z): 290[M+H] + , 1 H-NMR (DMSO): 2.85-2.89(t, 2H), 3.05-3.09(t, 2H), 6.68(s, 2H), 7.56(s, 1H), 8.37(s, 1H), 9.50(s, 1H)
Embodiment 2
[0084] 1-(6,8-Dichloro-4-thiochromanone)thiosemicarbazone
[0085] Dissolve 23.3g (0.1mol) of 6,8-dichloro-4-thiochromanone and 91g (0.1mol) of thiosemicarbazide in 100ml of ethanol, add dropwise 0.5ml of glacial acetic acid, heat to reflux for 5 hours, and cool After reaching room temperature, a solid precipitated out, was suction filtered, rinsed with a small amount of ethanol, and dried to obtain a crude product. The product obtained by recrystallization with n-butanol as a solvent is a white solid, the yield is 27.8g, the yield is 91%, mp: 174~176°C, LC-MS (m / z): 307[M+H] + , 1 H-NMR (DMSO): 2.92-2.94 (t, 2H), 2.97-2.99 (t, 2H), 7.52-7.53 (d, 1H), 8.28 (s, 1H), 8.36 (s, 1H), 8.43- 8.44(d, 1H), 10.25(s, 1H)
Embodiment 3
[0087] 1-[6,8-Dichloro-3-(N,N-dimethylamino)methyl-4-thiochromanone]semicarbazone hydrochloride
[0088] Method A:
[0089] 32.7g (0.1mol) of 6,8-dichloro-3-(N,N-dimethylamino)methyl-4-thiochromanone hydrochloride, 75g (0.1mol) of semicarbazide, 40.5g of sodium acetate (0.05mol) was dissolved in 100ml of ethanol, the pH of the reaction solution was adjusted to 3 with hydrochloric acid, heated to reflux for 6 hours, cooled to room temperature, a solid precipitated, filtered with suction, rinsed with a small amount of ethanol, and dried to obtain a crude product. The product obtained by recrystallization with n-butanol as a solvent is a white solid, the yield is 28.7g, the yield is 75%, mp: 207~209°C, LC-MS (m / z): 383[M+H] + , 1 H-NMR (DMSO): 2.22-2.27 (m, 6H), 2.50 (s, 2H), 2.61-2.65 (t, 2H), 2.75-2.80 (t, 2H), 6.78 (s, 2H), 7.59 ( s, 1H), 8.49(s, 1H), 10.10(s, 1H)
[0090] Method B:
[0091]29.1 g (0.1 mol) of 1-(6,8-dichloro-4-thiochromanone) semicarbazone prepared by me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com