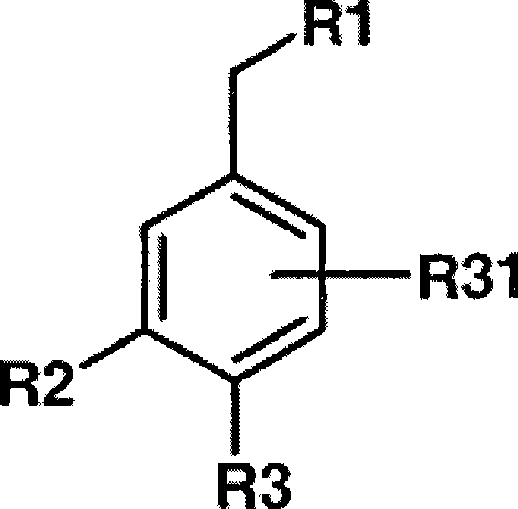
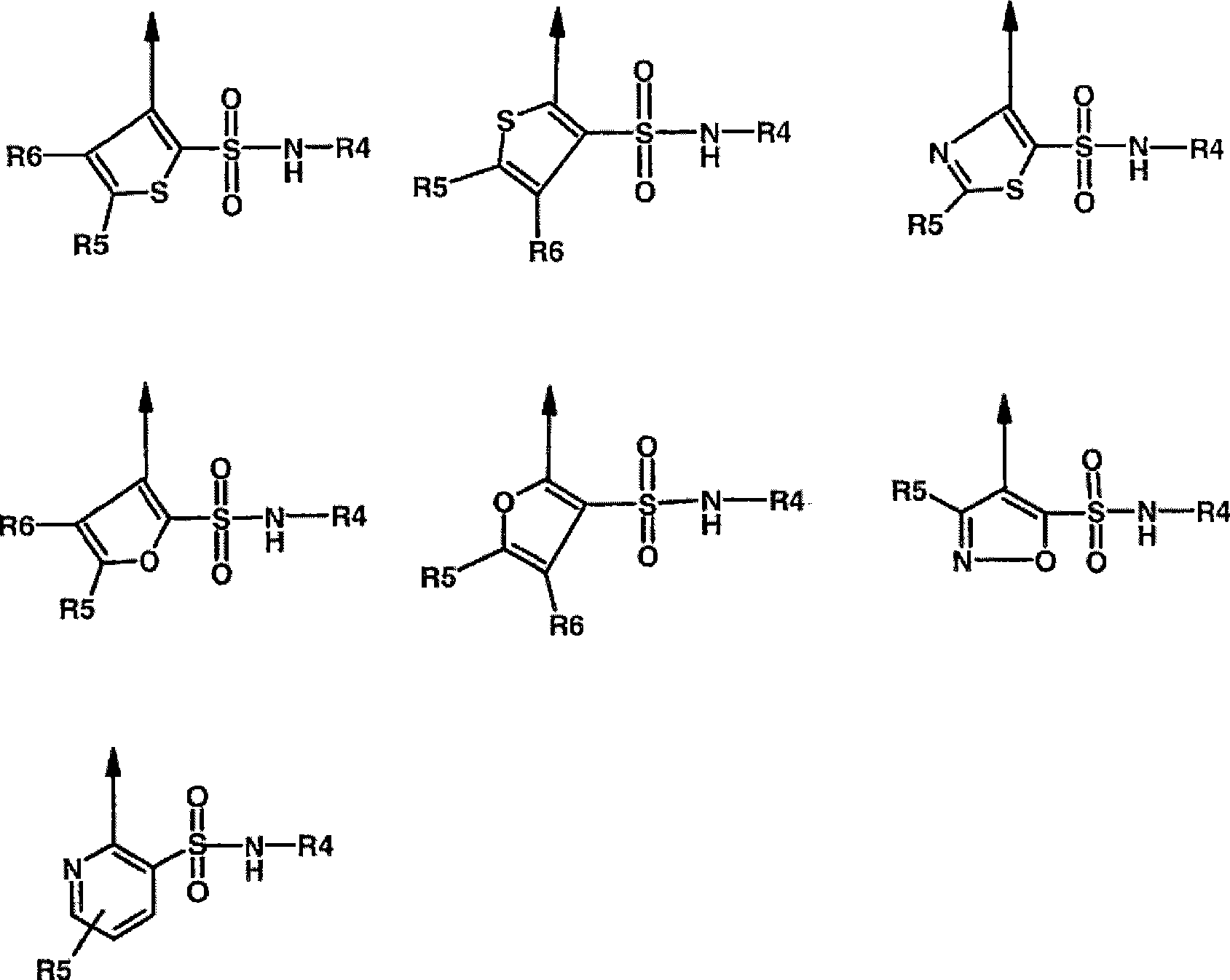
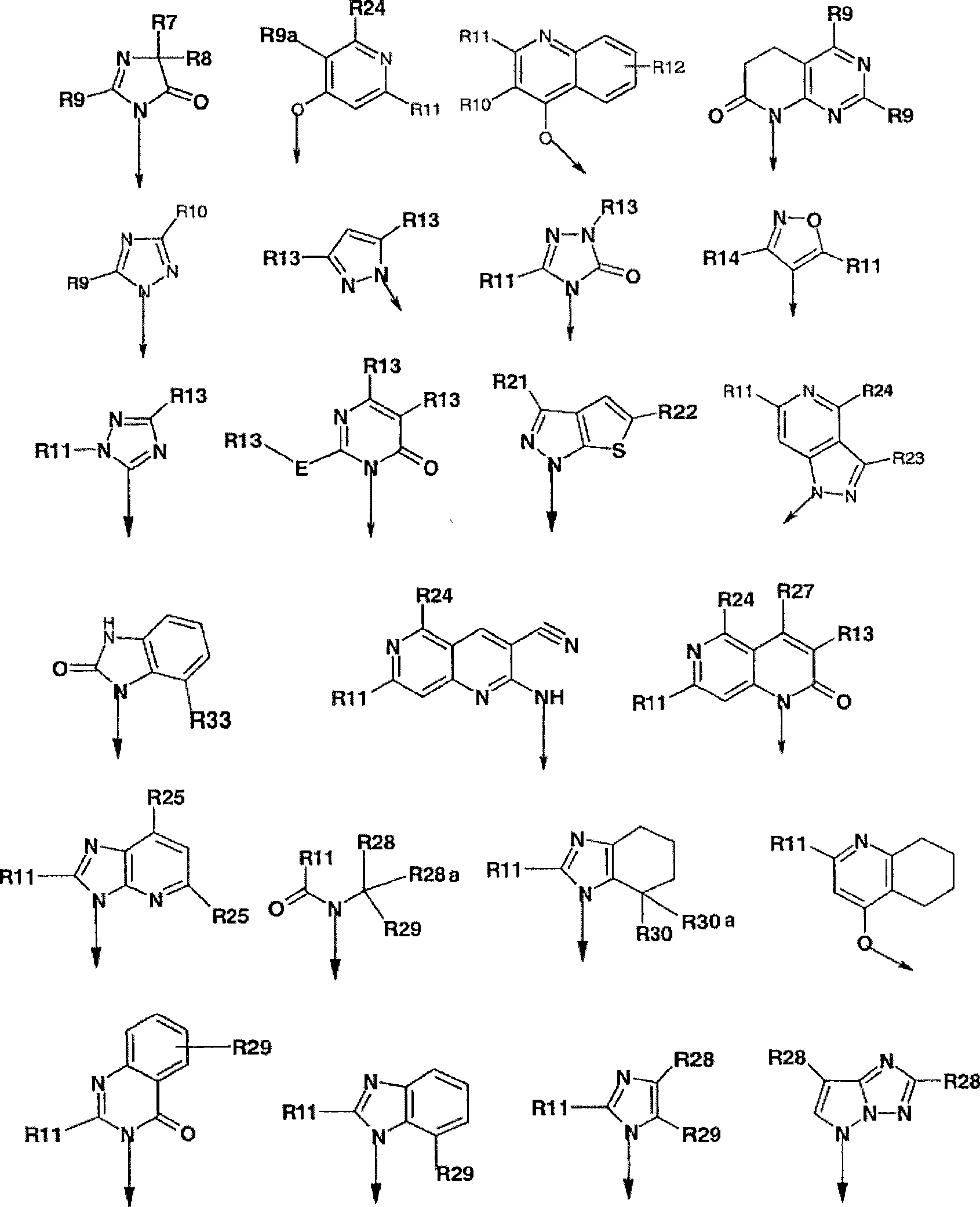
Novel dual action receptors antagonists (DARA) at the AT1 and ETA receptors
A technology of medicinal salts and enantiomers, which can be used in medical preparations containing active ingredients, antitumor drugs, allergic diseases, etc., and can solve the problem that the affinity of ETA cannot be zero.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0212]
[0213] 3-[4-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-ylmethyl)-2-ethoxymethyl-benzene base]-5-methyl-thiophene-2-sulfonic acid (4,5-dimethyl-isoxazol-3-yl)-amide
[0214] Step 01: Synthesis of ethyl 4-bromo-3-methylbenzoate
[0215]
[0216] To a cooled (0° C.) solution of 4-bromo-3-methylbenzoic acid (25 g, 0.116 mol) in (100 ml) ethanol was added (8 ml) concentrated sulfuric acid with stirring. After complete addition, the reaction mixture was refluxed for 6 hours. The reaction mixture was cooled, then concentrated in vacuo. Cold water (50 ml) was added to the residue (residue), followed by extraction with diethyl ether (100 ml×2). The organic layer was washed with saturated sodium bicarbonate solution followed by water and brine solution washes. Finally, the ether layer was dried over sodium sulfate and evaporated in vacuo to yield 26 g of ethyl 4-bromo-3-methylbenzoate.
[0217] Step 02: Synthesis of ethyl 4-bromo-3-(bromomethyl)benzoate
[02...
Embodiment 2
[0267]
[0268] 3-[4-(6-Ethyl-4-methyl-3-phenyl-pyrazolo[4,3-c]pyridin-1-ylmethyl)-phenyl]-thiophene-2-sulfonic acid (4,5-Dimethyl-isoxazol-3-yl)amide
[0269] Step 01: Synthesis of 1-phenyl-butane-1,3-dione
[0270]
[0271] Sodium ethoxide (13.5 g, 0.198 mol) was added to a stirred solution of dry ethyl acetate (80 ml, 0.72 mol) at -5°C. To this reaction mixture was added acetophenone (20 g, 0.185 mol) at -5°C, and then the temperature of the reaction mixture was maintained at 0°C for 12 hours. Then the reaction mixture was acidified with 1N hydrochloric acid and extracted with ethyl acetate (100ml×2). The combined organic layers were washed with water and brine. The organic layer was dried over sodium sulfate and evaporated to yield 21 g of 1-phenyl-butane-1,3-dione as a yellow solid.
[0272] Step 02: Synthesis of 3-amino-1-phenyl-but-2-en-1-one
[0273]
[0274] A mixture of 1-phenyl-butane-1,3-dione (20 g, 0.123 mol) and ammonium acetate (38 g, 0.49 mol...
Embodiment 3
[0325]
[0326] 3-[4-(5,7-diethyl-2-oxo-4-phenylsulfanyl)-2H-[1,6]naphthyridin (naphthyridin)-1 methyl Base)-phenyl]-thiophene-2-sulfonic acid (4,5-dimethyl-isoxazol-3-yl)-amide
[0327] Step 01: Synthesis of methyl 3-aminopentenoate
[0328]
[0329] A mixture of methyl propionoacetate (50 g, 0.3842 mol) and ammonium acetate (148 g, 1.921 mol) in dry methanol (500 mL) was stirred and refluxed for two days. The reaction mixture was cooled and concentrated in vacuo. The residue thus obtained was basified to pH 8 and extracted with ethyl acetate. The ethyl acetate layer was washed with water, brine, the organic layer was dried over sodium sulfate and concentrated to yield 50 g of methyl 3-aminopentenoate as a pale yellow liquid.
[0330] Step 02: Synthesis of methyl 2,6-diethyl-4-oxo-1,4-dihydro-pyridine-3-carboxylate
[0331]
[0332] To a mixture of methyl 3-aminopentenoate (50 g, 0.387 mol) and methyl propionoacetate (50 g, 0.384 mol) in o-xylene (200 ml) was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com