Multifunctional nanoparticle conjugates and their use
A technology of nanoparticles and conjugates, applied in the field of multifunctional nanoparticle conjugates and their applications, which can solve the problems that dispersion cannot be completely prevented
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0170] Synthesis of conjugated compounds
[0171] This example describes the paclitaxel-heparin-FA conjugate according to the process figure 1 Synthesis. Heparin (1 mmol) was activated overnight at 4°C with DCC (20 mmol) and NHS (22 mmol) in formamide. Dicyclohexylurea (DCU) was removed by filtration, and then heparin-NHS was obtained by recrystallization. Activated heparin-NHS (1 mmol) and aminated FA (20 mmol) were reacted again at room temperature for 1 day. FA was aminated with a conjugate with ethylenediamine. Dialysis removed unreacted aminated FA (molecular weight cutoff 2000). The yellowish final product was obtained by lyophilization. The yield of the conjugate was 95% (w / w). After the heparin-FA conjugate was dissolved in formamide (1 mmol), paclitaxel (30 mmol) and DCC (30 mmol) in DMSO were added. The mixture was reacted overnight at room temperature. After the reaction, recrystallization and filtration were performed to remove unreacted DCC. For further p...
Embodiment 2
[0176] Identification of conjugated compounds
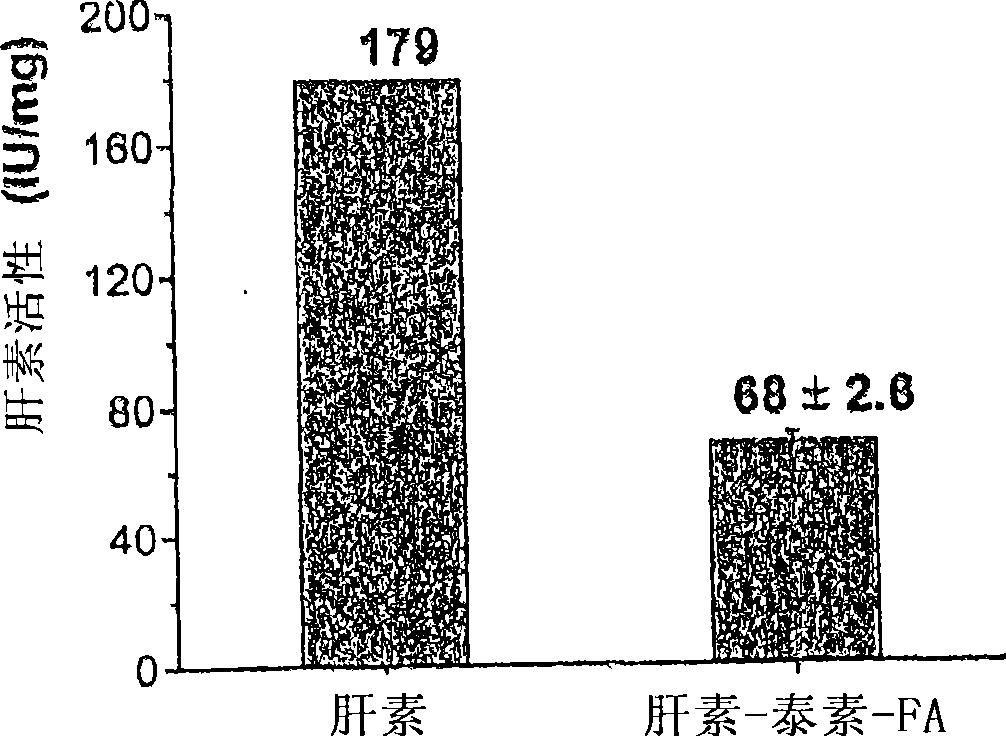
[0177] This example describes the identification of paclitaxel-heparin-FA produced in Example 1. UV-Vis absorption spectra were recorded on a Shimadzu UV-2401PC scanning spectrophotometer operating with a slit width of 1.0 nm. The content of paclitaxel conjugated to heparin-FA was estimated by UV measurement (λ = 228 nm) based on a standard curve generated with known concentrations of paclitaxel in methanol. IR spectra of paclitaxel-heparin-FA were acquired on a Fourier transform infrared spectrometer (FT-IR) with a Perkin Elmer system 2000 spectrometer and samples were analyzed as KBr pellets.
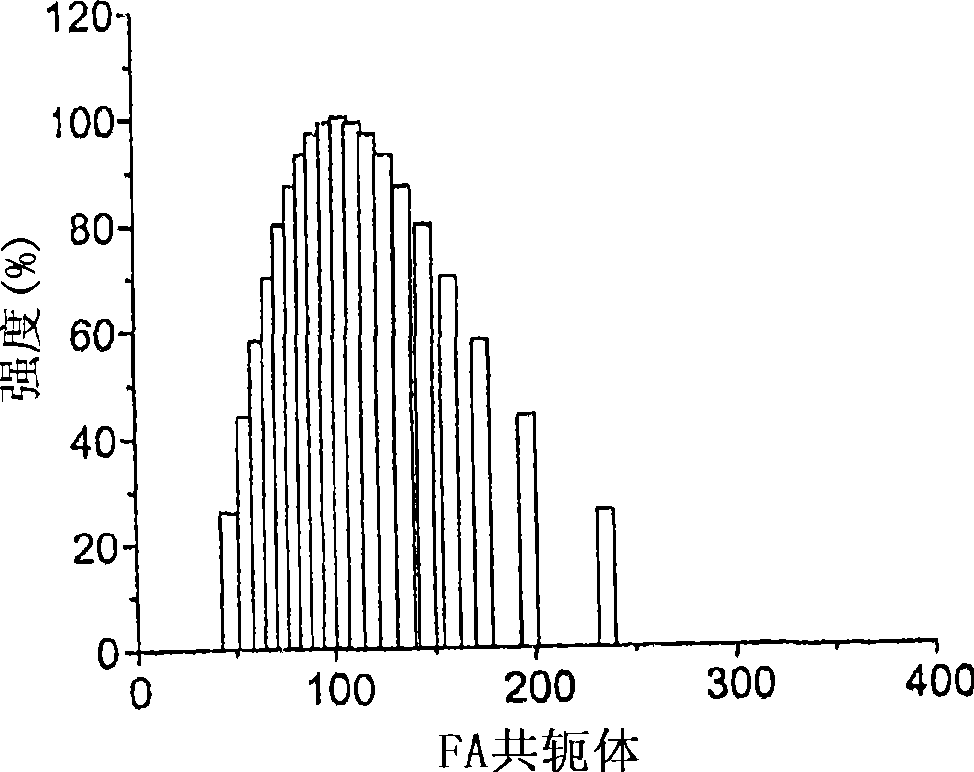
[0178] The synthesis of heparin-FA is through the 1 A signal was present at δ 6.75-8.77 ppm of the H-NMR spectrum and confirmed by absorption at λ = 280 nm of the UV spectrum of heparin-FA. The linkage between paclitaxel and heparin-FA is achieved through the DCC-mediated reaction between the hydroxyl group of paclitaxel and the carbo...
Embodiment 3
[0183] Identification of microtubule-binding activity
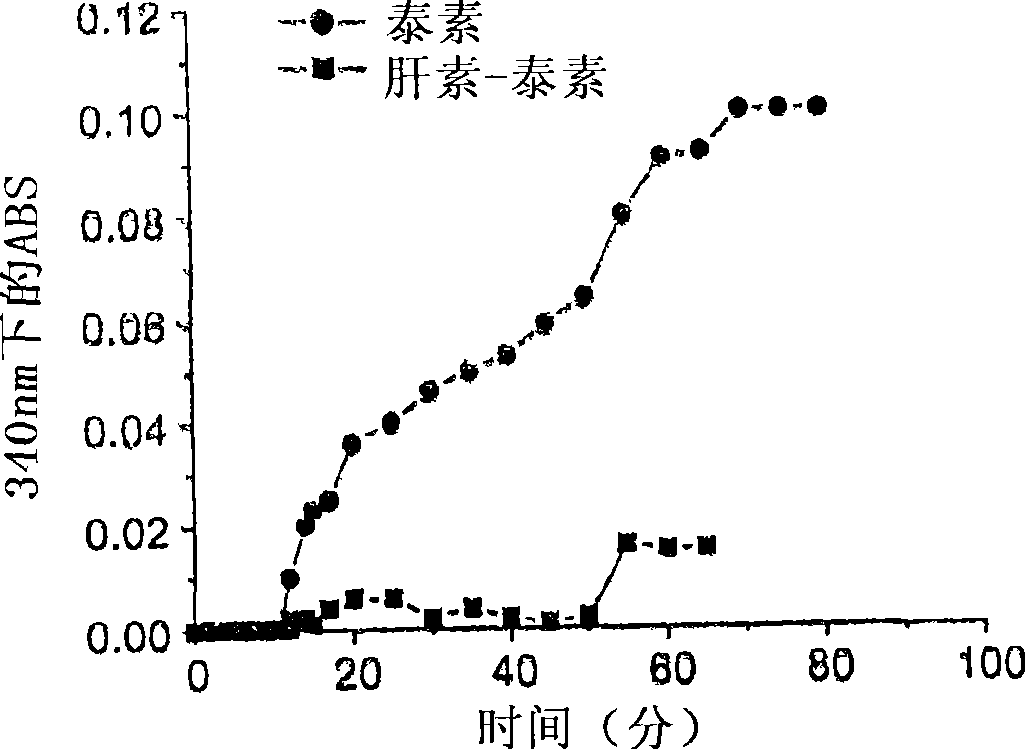
[0184] This example describes the microtubule polymerization assay used to evaluate compounds of the disclosure. Microtubule assembly reactions were performed in G-PEM buffer (1 mM GTP, 80 mM PIPES, 1 mM EGTA, 0.5 mM MgCl; pH 6.8) in the presence of drugs (10 μM) at concentrations of microtubule (Cytoskeleton Inc., Boulder, CO) It is 1mg / ml (10μM). The instrument was zeroed at 4°C with this solution. Paclitaxel or heparin-paclitaxel conjugates were then rapidly mixed into the microtube solution to a final concentration of 10 [mu]M, and absorbance was continuously monitored for a period of 80 minutes. Such samples were placed in quartz cuvettes and incubated at 32°C. Microtubule polymerization was observed by measuring the absorbance (340 nm) of the solution.
[0185] refer to image 3 , the ability of paclitaxel and paclitaxel-heparin-FA conjugates to induce microtubule assembly in vitro was determined at 10 μM pacli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com