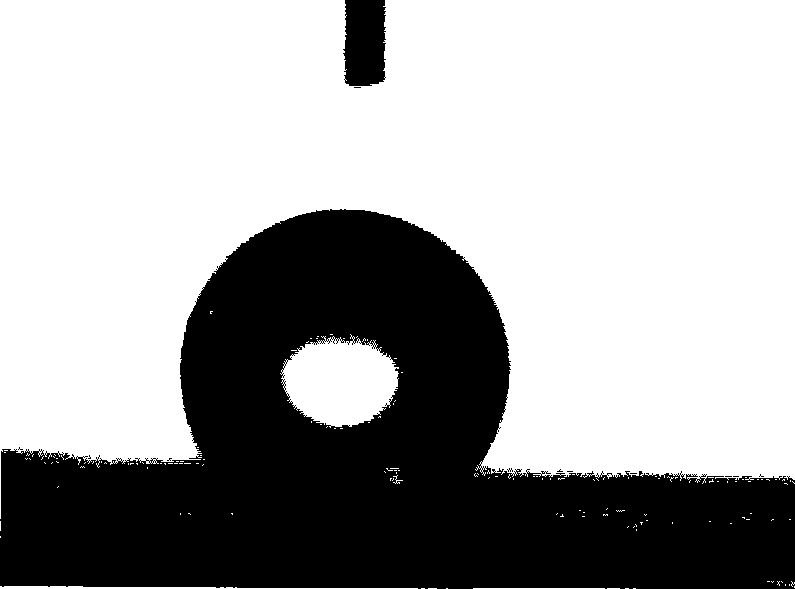Preparation of super-hydrophobic surface for metal anti-corrosive and self-cleaning effects
A super-hydrophobic surface, metal anti-corrosion technology, applied in the direction of electrolytic organic material coating, etc., can solve the problems of difficult process control, long preparation time, complicated operation, etc., and achieve the effect of low cost, simple operation, and high environmental stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
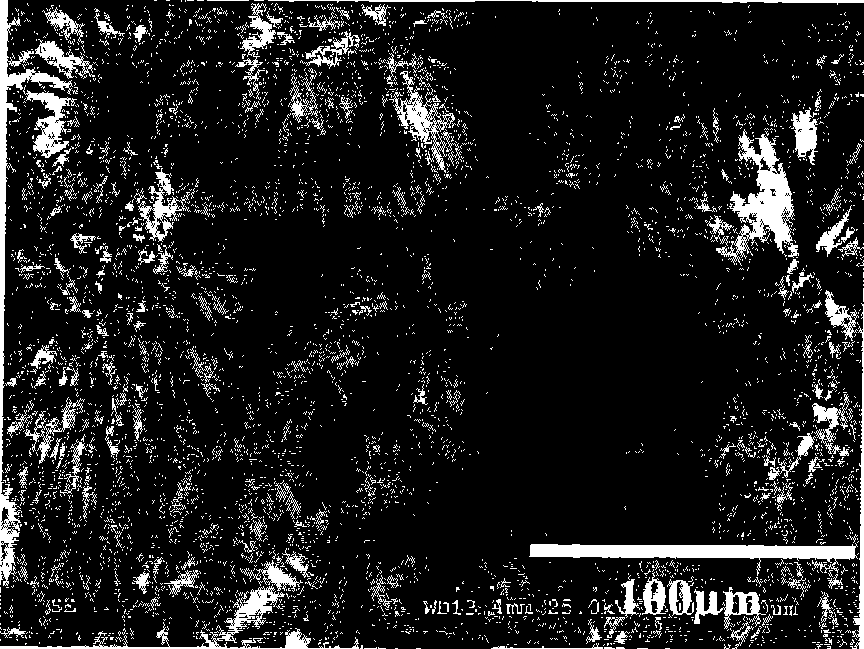
Image
Examples
Embodiment 1
[0027] 1. Use an ultrasonic cleaner to clean the metal copper sheet sample for the test. The cleaning steps are: put the copper substrate in acetone, deionized water, and ethanol in sequence and clean it with ultrasonic waves for 5 minutes each to remove the grease and other pollution on the surface substance;
[0028] 2. Use the treated copper sheet as the anode and cathode of the electrochemical reaction respectively, and put the 3 (CH 2 ) 10 COOH) in the ethanol solution, apply a DC voltage of 5V between the anode and the cathode;
[0029] After 3.1 hours, the cathode copper sheet was taken out, rinsed with ethanol and deionized water, and dried in the atmosphere to obtain a superhydrophobic surface layer attached to the surface of the cathode copper sheet.
[0030] 4. After testing with the contact angle detector, the results are: the contact angle is 154.8°, and the rolling angle is 2.3°. Such as image 3 As shown, the water droplet is approximately spherical on the p...
Embodiment 2
[0032] 1. Use an ultrasonic cleaner to clean the metal zinc and iron sheet samples used in the test. The cleaning steps are: put the metal zinc and iron substrates in acetone, deionized water, and ethanol in turn and clean them with ultrasonic waves for 5 minutes each to remove the surface. grease and other pollutants;
[0033]2. Use the treated metal zinc and iron substrates as the anode and cathode of the electrochemical reaction respectively and put in 0.05mol / L fatty acid (CH 3 (CH 2 ) 10 COOH) in the ethanol solution, apply a DC voltage of 2V between the anode and the cathode;
[0034] 3. After 2 hours, the cathode iron sheet is taken out, rinsed with ethanol and deionized water, and dried in the atmosphere to obtain a super-hydrophobic surface layer attached to the cathode iron sheet surface.
[0035] 4. After testing with the contact angle detector, the results are: the contact angle is 153.8°, and the rolling angle is 2.5°.
Embodiment 3
[0037] 1. Use an ultrasonic cleaner to clean the metal copper and aluminum samples for the test. The cleaning steps are as follows: put the copper substrate in acetone, deionized water, and ethanol in sequence and clean it with ultrasonic waves for 5 minutes each to remove the grease and oil on the surface. other pollutants;
[0038] a) Use the treated metal copper and aluminum substrates as the anode and cathode of the electrochemical reaction respectively and put 0.05mol / L fatty acid (CH 3 (CH 2 ) 12 COOH) in the ethanol solution, apply a DC voltage of 4V between the anode and the cathode;
[0039] 2. After 2 hours, take out the cathode aluminum sheet, rinse it with ethanol and deionized water, and dry in the atmosphere to obtain the super-hydrophobic surface layer attached to the surface of the cathode aluminum sheet.
[0040] 3. After testing with the contact angle detector, the results are: the contact angle is 157.2°, and the rolling angle is 2.1°.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Roll angle | aaaaa | aaaaa |
| Roll angle | aaaaa | aaaaa |
| Roll angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com