Application of polymerizable deep-eutectic solvents to preparing transparent conductive elastomers
A low eutectic solvent, transparent conductive technology, applied in the field of ionic liquids, can solve the problems of reduced conductivity, poor conductivity, high price of nanoparticles, and achieve the effects of low cost, low pollution and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A transparent conductive elastomer, the preparation process is as follows:
[0032] S1: Preparation of a polymerizable deep eutectic solvent: 13.96 g of hydrogen bond acceptor choline chloride and 14.41 g of hydrogen bond donor acrylic acid were stirred and reacted at 90° C. for 4 h to obtain a clear and transparent polymerizable deep eutectic solvent.
[0033] S2: Preparation of transparent conductive elastomer prepolymer mixed solution: 0.34g of polyethylene glycol diacrylate, 0.57g of 2-hydroxy-4'-(2-hydroxyethoxy)-2-methylpropiophenone Mix evenly with the deep eutectic solvent, stir and react for 2 hours to obtain a transparent conductive elastomer prepolymer mixed solution.
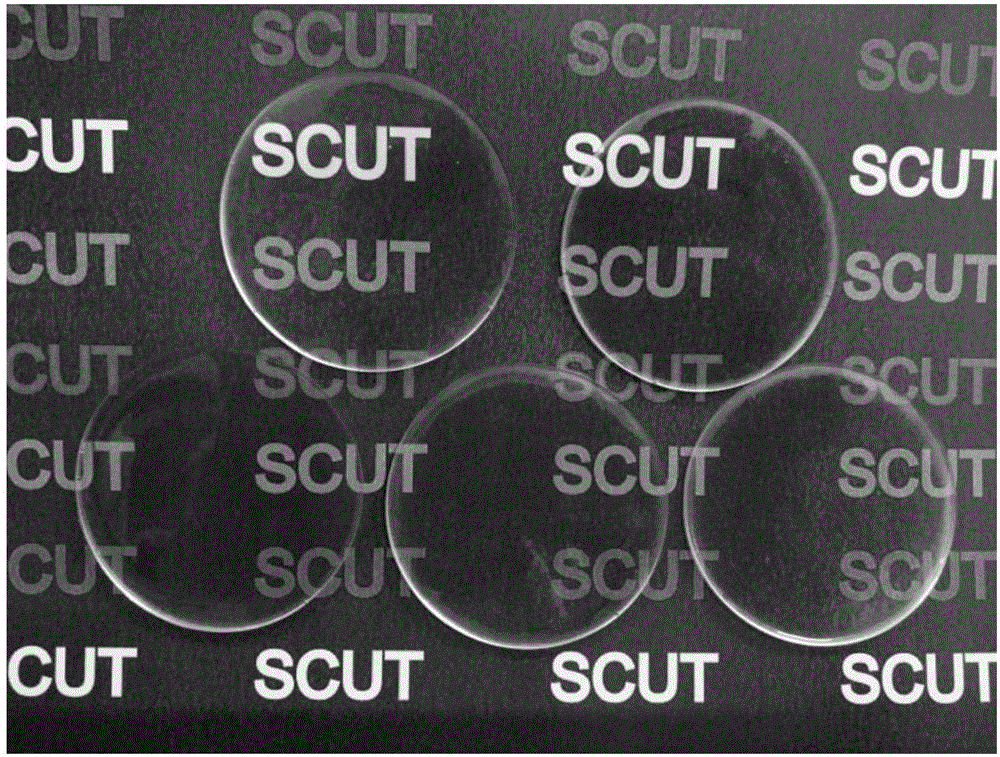
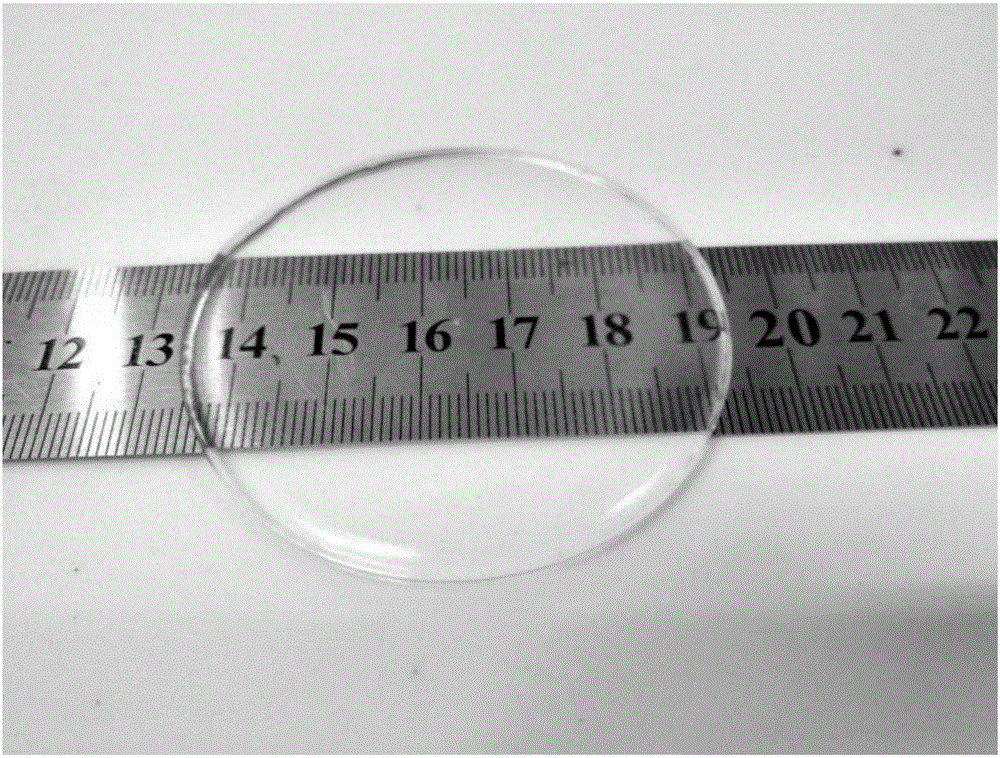
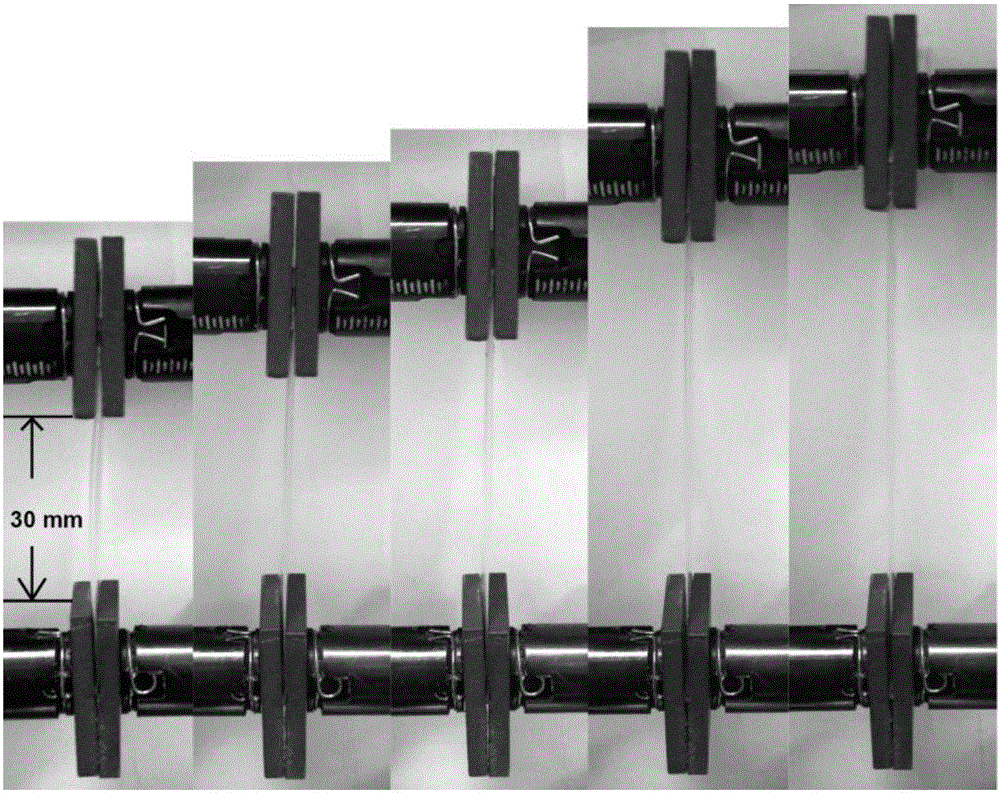
[0034] S3: Preparation of conductive elastomer: take 2.83ml of the transparent conductive elastomer prepolymer mixed solution described in step S2, pour it into a polytetrafluoroethylene petri dish (radius 3cm), and cure it under ultraviolet light (2Kw) for 8s to obtain a transparent conducti...
Embodiment 2
[0036] A transparent conductive elastomer, the preparation process is as follows:
[0037] S1: Preparation of a polymerizable deep eutectic solvent: 13.96 g of hydrogen bond acceptor choline chloride and 14.41 g of hydrogen bond donor acrylic acid were stirred and reacted at 90° C. for 4 h to obtain a clear and transparent polymerizable deep eutectic solvent.
[0038] S2: Preparation of transparent conductive elastomer prepolymer mixed solution: 0.68g of polyethylene glycol diacrylate, 0.58g of 2-hydroxy-4'-(2-hydroxyethoxy)-2-methylpropiophenone Mix evenly with the deep eutectic solvent, stir and react for 2 hours to obtain a transparent conductive elastomer prepolymer mixed solution.
[0039] S3: Preparation of conductive elastomer: take 2.83ml of the transparent conductive elastomer prepolymer mixed solution described in step S2, pour it into a polytetrafluoroethylene petri dish (radius 3cm), and cure it under ultraviolet light (2Kw) for 8s to obtain a transparent conducti...
Embodiment 3
[0041] A transparent conductive elastomer, the preparation process is as follows:
[0042] S1: Preparation of a polymerizable deep eutectic solvent: 13.96 g of hydrogen bond acceptor choline chloride and 14.41 g of hydrogen bond donor acrylic acid were stirred and reacted at 90° C. for 4 h to obtain a clear and transparent polymerizable deep eutectic solvent.
[0043]S2: Preparation of transparent conductive elastomer prepolymer mixed solution: 1.02 of polyethylene glycol diacrylate, 0.59 g of 2-hydroxy-4'-(2-hydroxyethoxy)-2-methyl propiophenone and The deep eutectic solvent was mixed evenly, and stirred and reacted for 2 hours to obtain a transparent conductive elastomer prepolymer mixed solution.
[0044] S3: Preparation of conductive elastomer: take 2.83ml of the transparent conductive elastomer prepolymer mixed solution described in step S2, pour it into a polytetrafluoroethylene petri dish (radius 3cm), and cure it under ultraviolet light (2Kw) for 8s to obtain a transpa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com