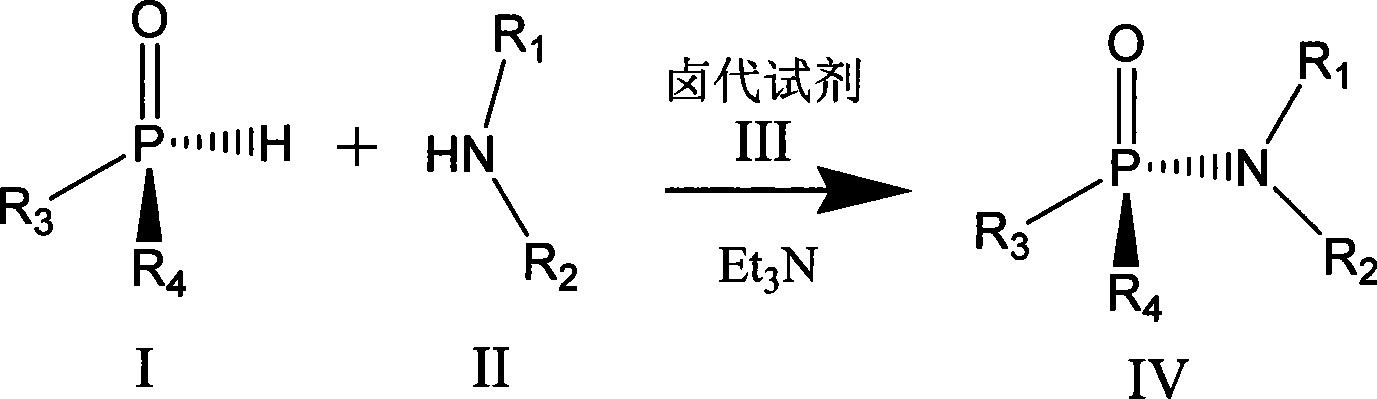
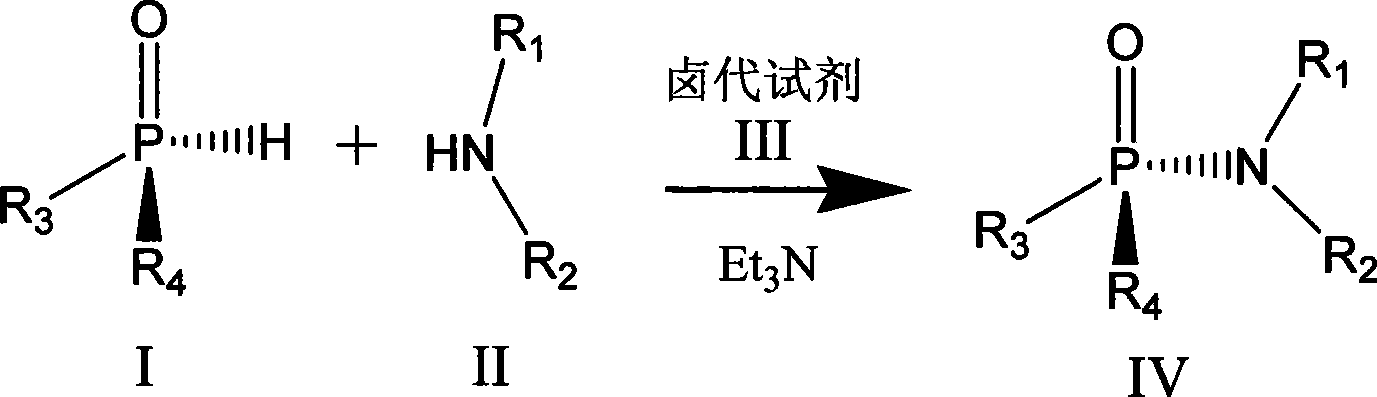
Chiral phosphamide synthesizing method
A synthesis method and phosphoramide technology, which is applied in the field of synthesizing chiral phosphoramides with a single configuration of the phosphorus chiral center, can solve the problems of difficult operation and waste, and achieve the effects of easy acquisition, mild reaction conditions, and low raw material prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a 25mL round-bottomed flask, put 0.5mmol (Rp) menthoxyphenyl hydrogen phosphite and 30mg ammonia water in 10mL acetonitrile, keep the temperature at 0°C, and finally add 0.5mmol of C 2 Cl 6 Dissolve the solution in 5ml of acetonitrile and continue to stir overnight. After the reaction is over, remove the solvent under reduced pressure to obtain a crude product. Purification by column chromatography was carried out under the condition of eluent whose polarity was petroleum ether: ethyl acetate = 5:1-1:2 to obtain the product with a yield of 75%.
Embodiment 2
[0023] In a 25mL round-bottomed flask, dissolve 0.5mmol (Rp) menthyl isopropoxyhydrogen phosphite and 1mmol phenylalanine in 5mL of water, add 5mL of acetonitrile, keep the temperature within 8°C, and then add Add 1 equivalent of triethylamine, and finally add 20 mmol of CCl dropwise 4 , continue to stir overnight, after the reaction is over, remove acetonitrile and other low-boiling organic matter under reduced pressure, remove unreacted menthyl isopropoxyhydrogen phosphite with 10 mL of dichloromethane, and use 1mol.L -1 dilute hydrochloric acid to adjust the pH value to about 3, dichloromethane 10mL extraction to obtain the crude product, the polarity of petroleum ether: ethyl acetate = 5:1 ~ 1:2 eluent conditions for column chromatography separation and purification, The product was obtained with a yield of 70%.
Embodiment 3
[0025] In a 25mL round bottom flask, 0.5mmol (Rp) menthoxybutoxyhydrogen phosphite and 1mmol diethylamine were dissolved in 10mL dichloromethane, the temperature was kept at -40°C, and then 1 equivalent of tris Ethylamine, finally dropwise the dichloromethane solution of the diisopropylamino chloride of 1mmol, react 3 hours after completion of dropwise addition, after reaction finishes, remove low-boiling point organic matter under reduced pressure, at polarity is sherwood oil: ethyl acetate= Purification by column chromatography was carried out under the condition of 5:1-1:2 eluent to obtain the product with a yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com