Use of black mulberry extract as tyrosinase inhibitor
A technology of tyrosinase and compounds, applied in the field of natural compounds, can solve problems such as lack of preservation methods and economic losses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
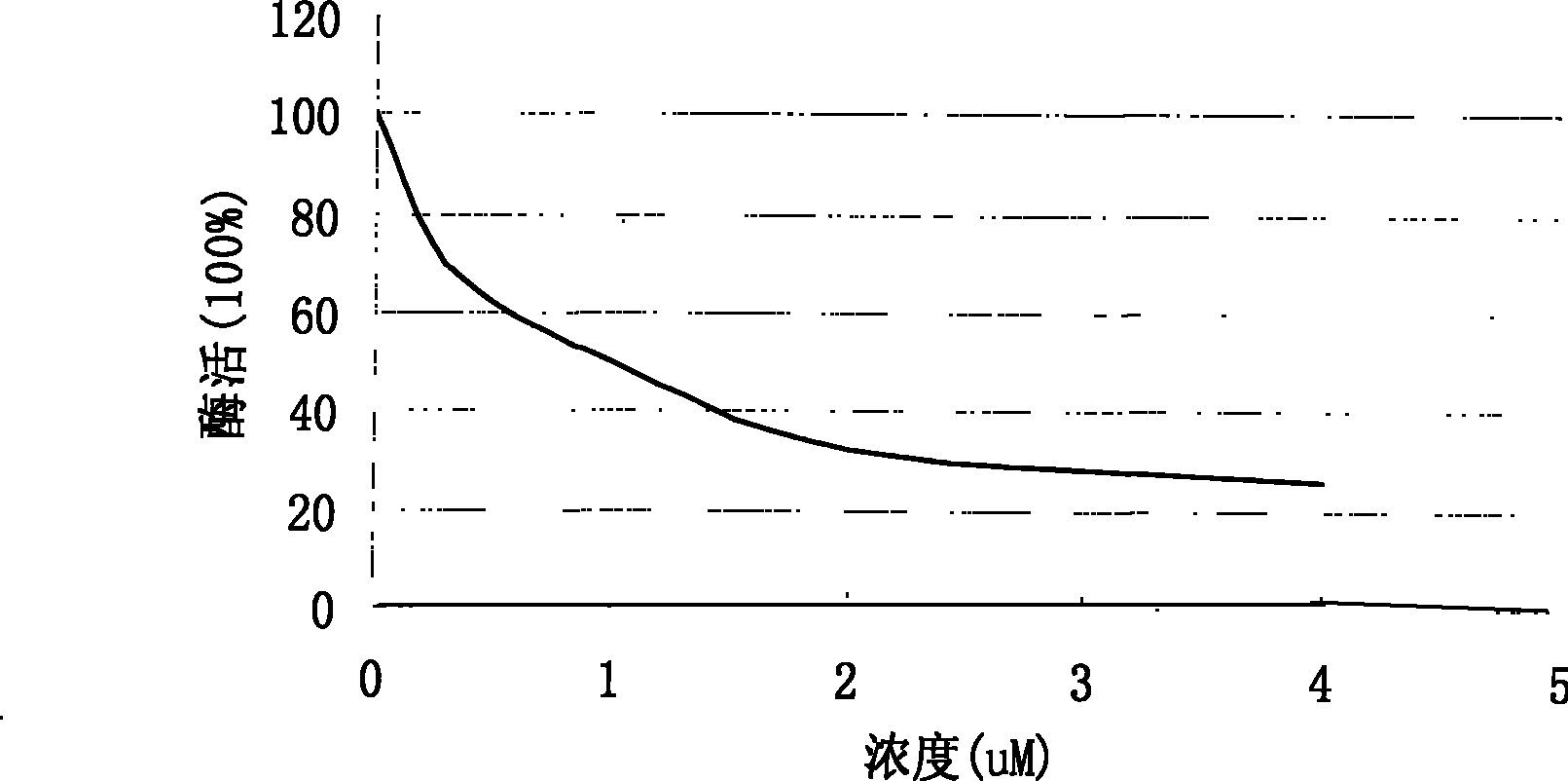
[0025] Example 1: Determination of compound (I) tyrosinase inhibitory activity.
[0026] The determination of tyrosinase inhibitory activity takes kojic acid as a reference substance, and the method is as follows:
[0027] The substrate dopa was first dissolved in phosphate buffer (25 mM, pH 6.8) to a concentration of 8 mM. To a 96-well plate, 20 μL of the above substrate solution, 90 μL of phosphate buffer, and 20 μL of compounds of different concentrations were added. Add 70 μL of tyrosinase-containing phosphate buffer (100 U / mL, pH 6.8) to start the reaction, and react at 37° C. for 10 minutes. Before and after the reaction, the absorbance of each well was measured with a Multiscan MK3 automatic multifunctional microplate reader (Thermo) at 492 nm.
[0028] According to the absorbance at 492nm, the inhibitory rate (%) of the compound to tyrosinase is calculated, and the concentration of the inhibitor when the enzyme activity inhibitory rate (%) reaches 50% is determined a...
Embodiment 2
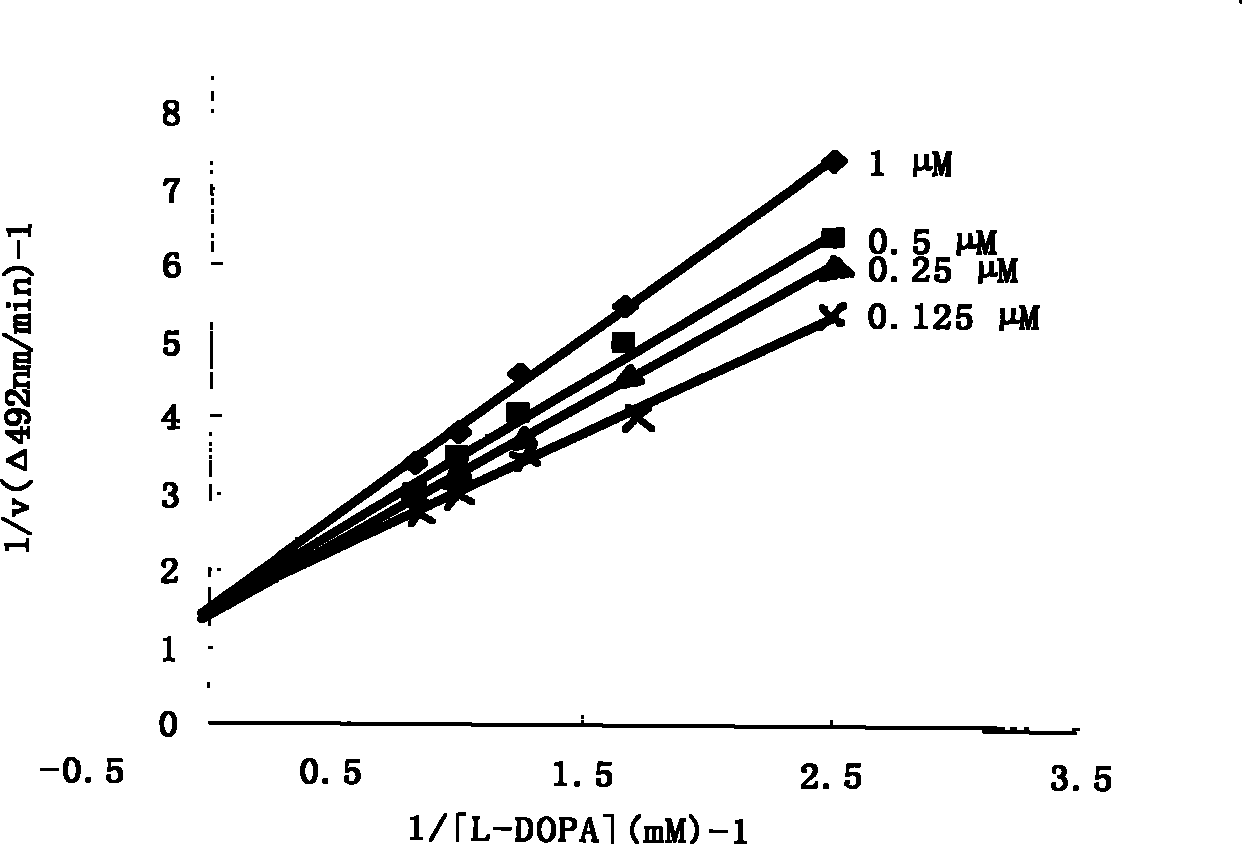
[0038] Example 2: Kinetic analysis of tyrosinase inhibition.
[0039] The substrate dopa was dissolved in phosphate buffer (25mM, pH6.8) and prepared into 4 concentrations of 12mM, 10mM, 8mM and 6mM. Into the 96-well plate, 20 μl of the above-mentioned substrate solutions of different concentrations, 90 μl of phosphate buffer, and 20 μl of compounds of different concentrations were added. Add 70 μL of tyrosinase-containing phosphate buffer (100 U / mL, pH 6.8) to start the reaction, and react at 37° C. for 10 minutes. Before and after the reaction, the absorbance of each well was measured at 492 nm. Calculate enzyme inhibition rate, method is with embodiment 1, draws Lineweaver-Burk curve (see figure 2 ).
[0040] Conclusion: from figure 2 It can be seen from the Lineweaver-Burk curve that compound (I) is a competitive inhibitor.
Embodiment 3
[0041] Example 3: Time Analysis of Tyrosinase Inhibition.
[0042] The substrate dopa was first dissolved in phosphate buffer (25 mM, pH 6.8) to a concentration of 8 mM. To a 96-well plate, add 20 μL of the above substrate solution, 90 μL of phosphate buffer, and 20 μL of the compound at a final concentration of 4 μM. Add 70 μL of tyrosinase-containing phosphate buffer (100 U / mL, pH 6.8) to start the reaction, and measure the absorbance of each well at different times. Calculate enzyme inhibition rate, method is the same as embodiment 1.
[0043] The results are shown in Table 2.
[0044] Table 2: Tyrosinase inhibitory activity at different times
[0045] time (min) Inhibition rate(%) 5 60.69±3.32 10 70.71±0.30 15 74.62±2.19 30 73.11±2.42 60 69.47±2.77 90 68.97±3.05 120 66.80±3.42 150 65.88±3.43 180 62.82±2.47
[0046] Conclusion: Compound (I) had an enzyme inhibition rate of 74.62% when reacted for 15 minutes. A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com