Glucagon-like peptide 1(glp-1) pharmaceutical formulations
A GLP-1, drug technology, applied in the field of pulmonary delivery pharmaceutical preparations, dry powder preparations, and pharmaceutical preparations for the treatment of diseases, can solve problems such as unavoidable side effects such as weight gain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118] Biophysical and analytical analysis of GLP-1 structure
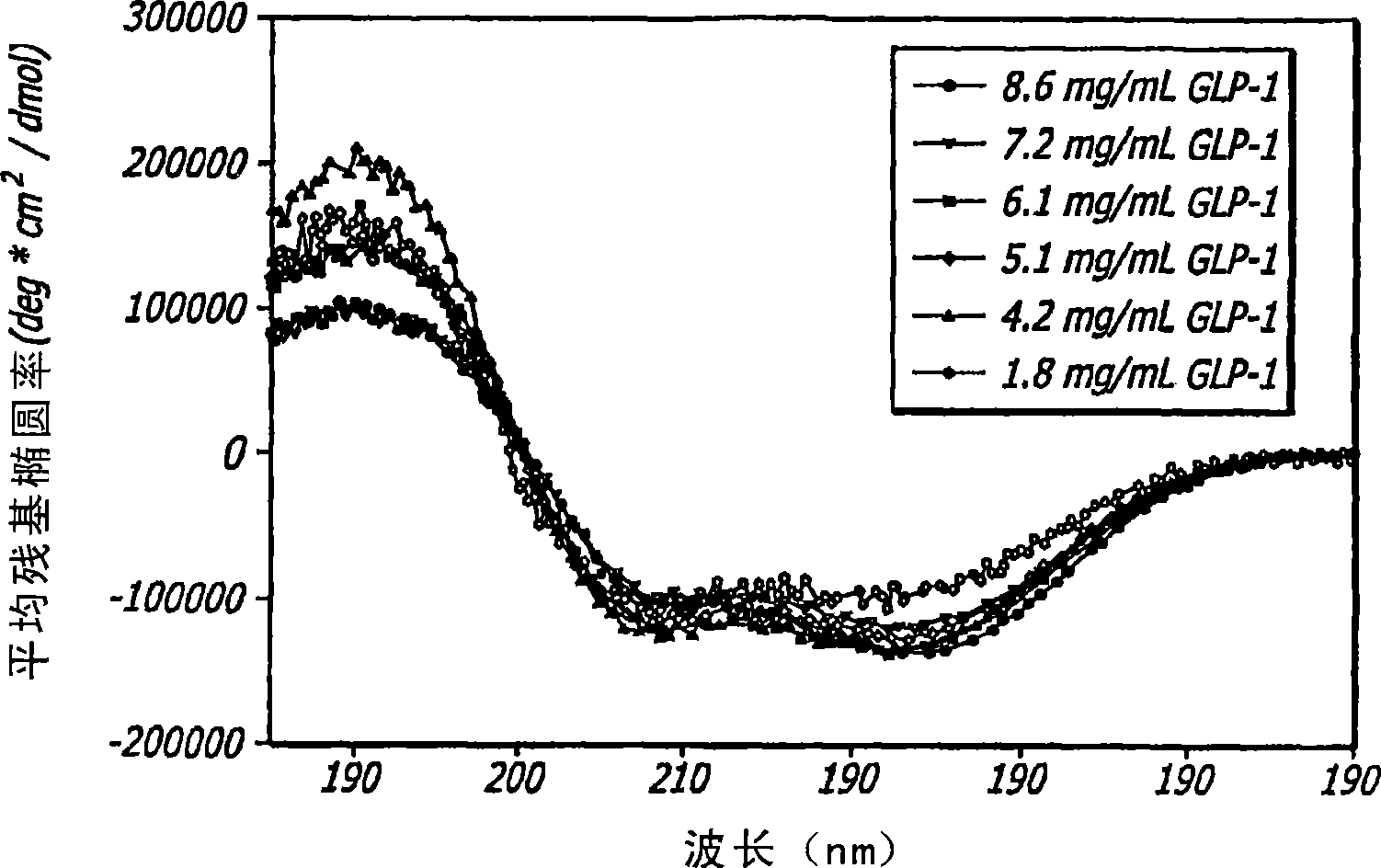
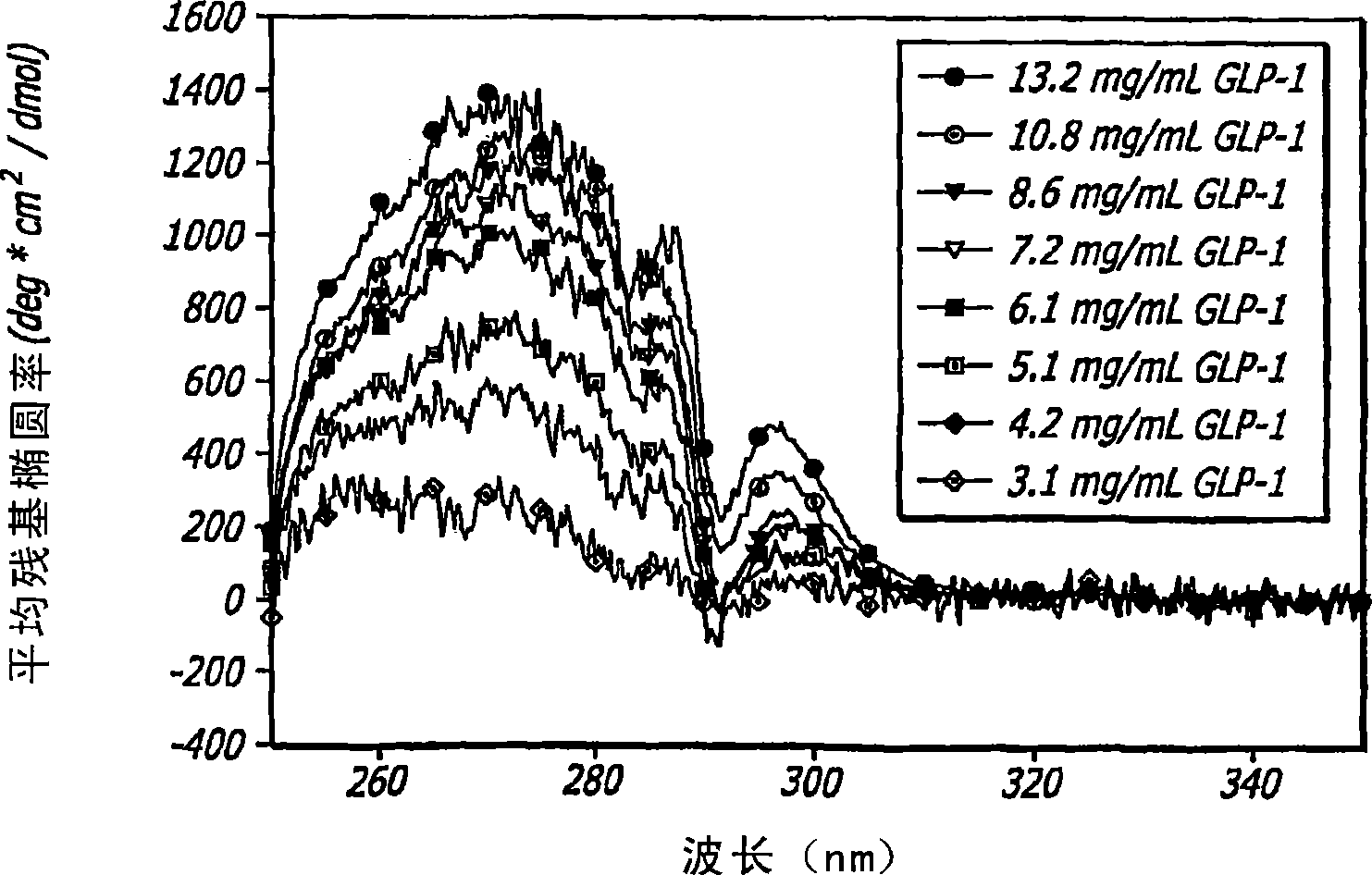
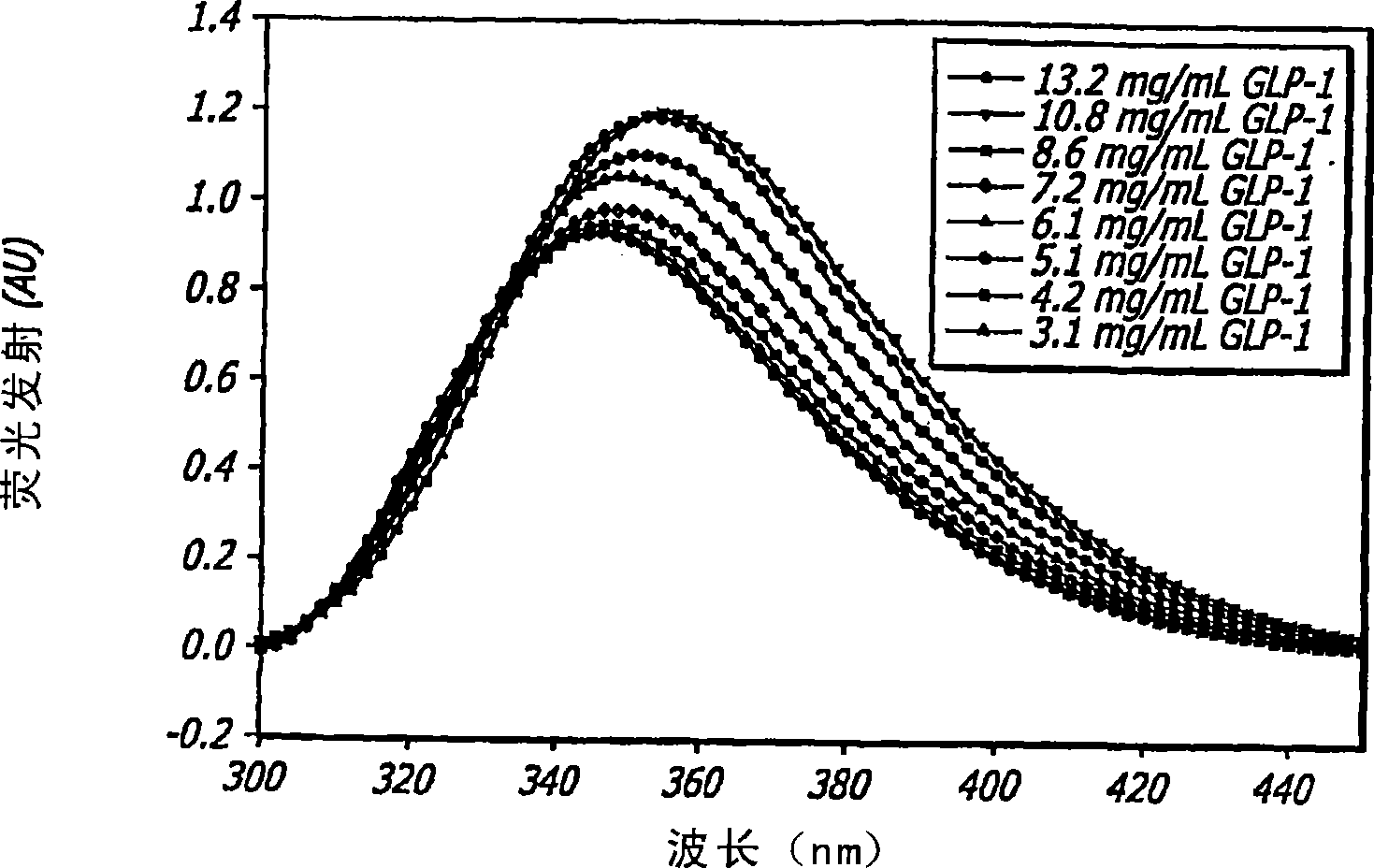
[0119] To analyze the structure and behavior of GLP-1, a large number of biophysical and analytical techniques are used. These techniques include far-ultraviolet circular dichroism (far-UV CD), near-ultraviolet circular dichroism (near-UV CD), internal fluorescence, Fourier transform infrared spectroscopy (FTIR), high-pressure liquid chromatography (HPLC), and mass spectrometry (MS); all of which are well known to those of ordinary skill in the art. The effects of concentration, ionic strength, temperature, pH, oxidative stress, agitation and multiple freeze-thaw cycles on GLP-1 peptides were studied using a wide range of conditions; all described in more detail below. These assays were also used to characterize major pathways of degradation and to establish conditions for manipulating the peptide structure of GLP-1 to achieve a certain GLP-1 / DKP formulation.
[0120] Experimental procedure
[0121] GLP-1 w...
Embodiment 2
[0144] GLP-1 / FDKP Adsorption Study
[0145] The interaction of GLP-1 with diketopiperazine (DKP) particles in suspension was evaluated by performing adsorption studies. Variables in adsorption studies explored the effects of electrostatics, hydrogen bonding, water structure, protein flexibility, and specific salt-pairing interactions on GLP-1 / DKP interactions. In addition, the effect of several common protein stabilizers on the surface adsorption of GLP-1 and DKP was tested.
[0146]Using preformed DKP suspension particles (ie, FDKP), the conditions under which GLP-1 adsorbed to the surface of preformed DKP particles were investigated. The FDKP particle suspension (where the FDKP particles were pre-formed) was combined with a solution of 3X pH buffer and 3X additive or excipient. The final solution had a FDKP concentration of 5, mg / ml and a GLP-1 concentration of 0.25, mg / ml (5% w / w). Unbound GLP-1 in the supernatant was filtered from the suspension. FDKP particles were ...
Embodiment 3
[0168] Integrity Analysis of GLP-1 / FDKP Preparations
[0169] Based on the results from the experiments in Examples 1 and 2, a series of GLP-1 preparations with the characteristics described in Table 1 were selected for the cell viability assays discussed herein. Most of the formulations contained GRAS ("Generally Regarded As Safe") excipients, but some were selected to allow studies of the relationship between stability and adsorption.
[0170] Table 1. GLP-1 / FDKP selected for Integrity Phase Analysis.
[0171]
[0172] Additionally, based on the results obtained in Examples 1 and 2, a series of formulations were also selected for Phase II integrity studies of GLP-1 / FDKP. Table 2 below shows the six GLP-1 formulations selected for Phase II integrity. After powders were prepared, they were blended with blank FDKP to produce similar amounts of GLP-1 peptide and FDKP in each formulation.
[0173] Table 2. GLP-1 / FDKP formulations selected for phase II integrity. Formulat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com