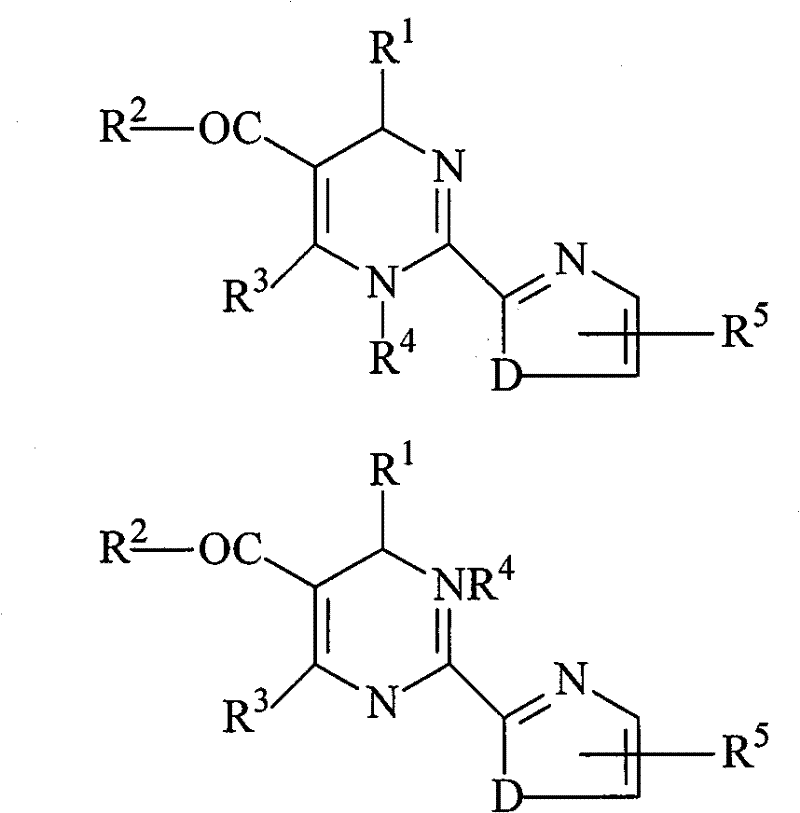
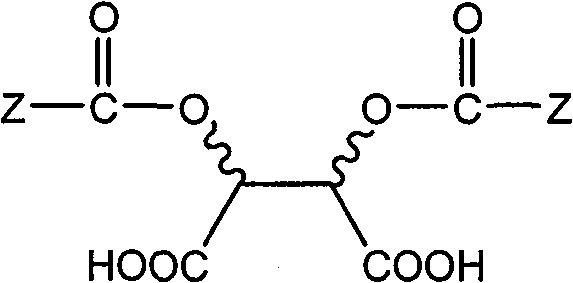
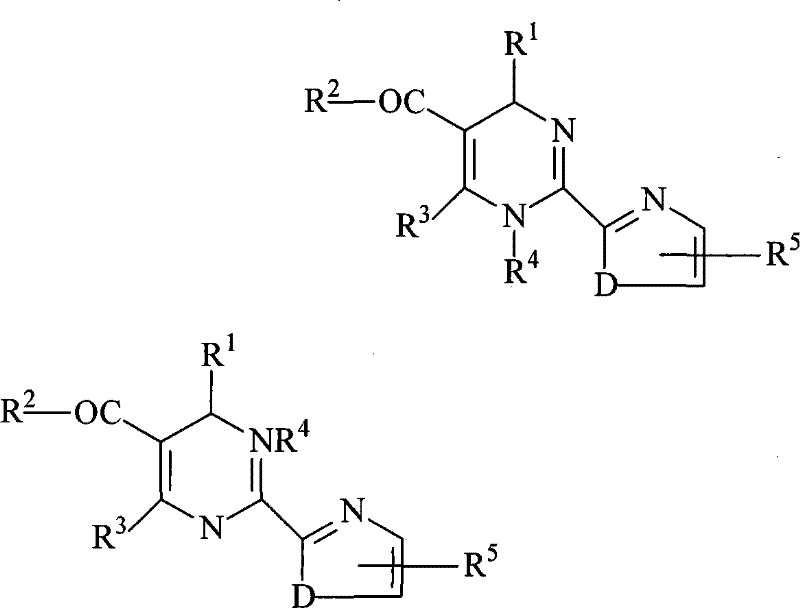
Method for splitting dihydropyrimidine racemic compound
A technology for dihydropyrimidines and compounds, which is applied in the field of resolution of organic racemic compounds, can solve the problems of high cost and difficulty in industrialization of resolution methods, and achieve the effects of enhanced diastereomer recognition, simple operation and high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) 1.0mol 4-(2-chlorophenyl)-6-methyl-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylate racemic compound , its structural formula is as follows:
[0036]
[0037] and 0.50mol D-p-methyl-dibenzoyl tartaric acid (D-(+)-DTTA) into the reactor, followed by adding 2.5L dichloromethane and 2.5L petroleum ether under mechanical stirring;
[0038] (2) Stir at 23°C for 0.5h, rotate left and right methyl 4-(2-chlorophenyl)-6-methyl-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylate Fully combined with D-p-methyldibenzoyl tartaric acid to form L-4-(2-chlorophenyl)-6-methyl-2-(thiazol-2-yl)-1,4-dihydropyrimidine- Methyl 5-carboxylate D-p-methyldibenzoyl tartrate and D-4-(2-chlorophenyl)-6-methyl-2-(thiazol-2-yl)-1,4- Methyl dihydropyrimidine-5-carboxylate D-p-methyldibenzoyl tartrate;
[0039] (3) filtering, concentrating the mother liquor to 0.2 times the original volume, and then placing the concentrated mother liquor at 0°C overnight;
[0040] (4) Filter the mother ...
Embodiment 2
[0043] (1) 1.0mol 4-(2-bromo-4-fluorophenyl)-6-methyl-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylic acid ethyl ester The racemic compound, its structural formula is as follows:
[0044]
[0045] and 1.0mol L-p-methyldibenzoyl tartaric acid (L-(-)-DTTA) into the reactor, followed by adding 4.5L dichloromethane and 32L petroleum ether;
[0046](2) Stir at 22°C for 1.0h, rotate 4-(2-bromo-4-fluorophenyl)-6-methyl-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5 -Ethyl carboxylate is fully combined with the resolving agent of L-p-methyldibenzoyl tartaric acid to form L-4-(2-bromo-4-fluorophenyl)-6-methyl-2-(thiazole-2- Base)-1,4-dihydropyrimidine-5-carboxylic acid ethyl ester L-p-methyldibenzoyl tartrate and D-4-(2-bromo-4-fluorophenyl)-6-methyl-2 -(Thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylic acid ethyl ester L-p-methyldibenzoyl tartrate;
[0047] (3) Filtration, concentrating the mother liquor to 0.5 times the original volume, and then placing the concentrated mother liq...
Embodiment 3
[0051] (1) 0.40mol 4-(2-bromo-4-fluorophenyl)-6-methyl-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylic acid ethyl ester The racemic compound and 1.2mol L-p-methoxydibenzoyl tartaric acid (L-(-)-DATA) are dropped into the reactor, and 2.5L acetone and 50L cyclohexane are added successively;
[0052] (2) Stir at 21°C for 3 hours, rotate 4-(2-bromo-4-fluorophenyl)-6-methyl-2-(thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylate Ethyl acetate is fully combined with L-p-methoxydibenzoyl tartaric acid resolving agent to form L-4-(2-bromo-4-fluorophenyl)-6-methyl-2-(thiazol-2-yl )-1,4-Dihydropyrimidine-5-carboxylic acid ethyl ester L-p-methoxydibenzoyl tartrate and D-4-(2-bromo-4-fluorophenyl)-6-methyl-2 -(Thiazol-2-yl)-1,4-dihydropyrimidine-5-carboxylic acid ethyl ester L-p-methoxydibenzoyl tartrate;
[0053] (3) filtering, concentrating the mother liquor to 0.6 times of the original volume, and then placing the concentrated mother liquor at room temperature for 48 hours;
[005...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com