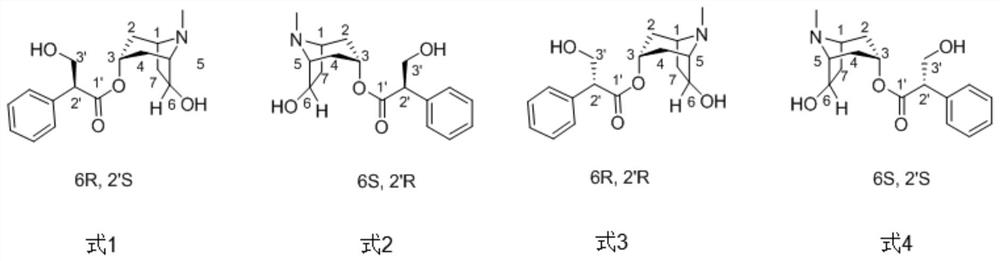
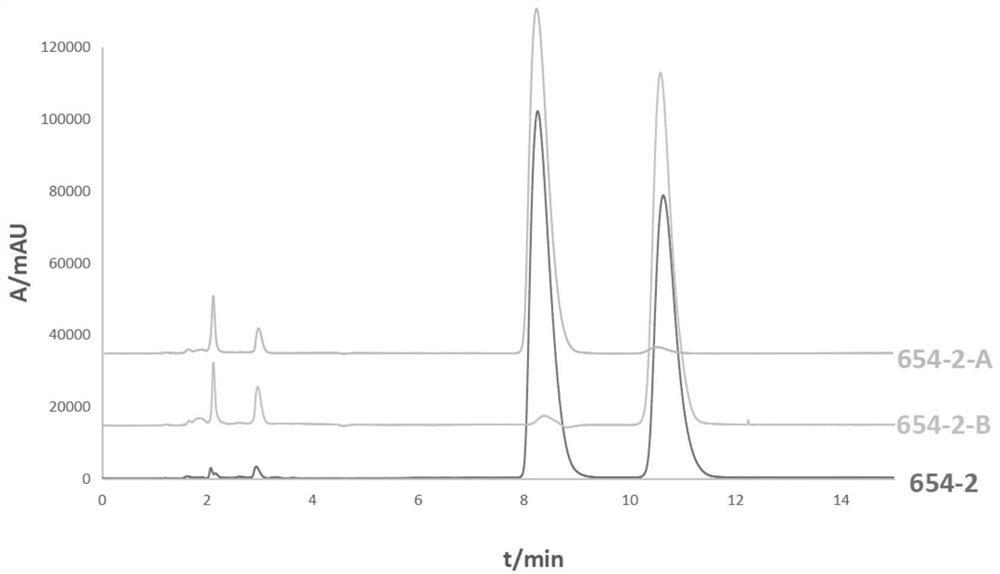
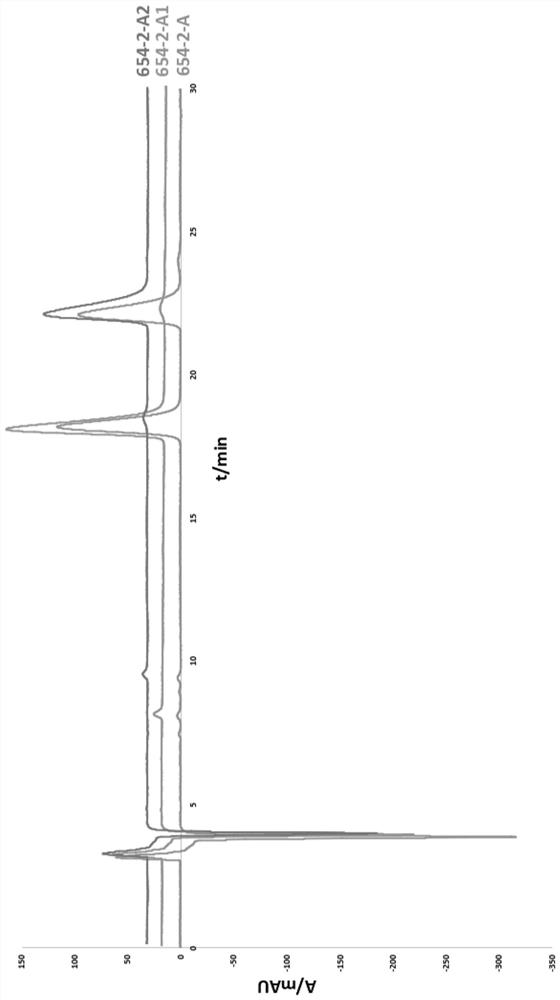
A kind of chiral separation method of four isomers in racemic anisodamine
An anisodamine and chiral separation technology, applied in the field of medicine, can solve the problems of being unable to be suitable for industrialized large-scale production, small preparation amount, long time-consuming and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The chiral resolution of four isomers in embodiment 1 racemic anisodamine
[0027] 1. Sample preparation
[0028] The racemic anisodamine sample (sample concentration: 100 mg / mL) was completely dissolved in an aqueous solution containing 45% (v / v) methanol, passed through a 0.22 μm microporous membrane for use.
[0029] 2. HPLC chromatographic separation
[0030] Mobile phase: a mixed solution of methanol, water and diethylamine, wherein the volume ratio of methanol:water:diethylamine=45:55:0.02.
[0031] Chromatographic conditions: the detection wavelength is 210nm, the chromatographic column is an Agilent Prep C18 column (250mm×9.4mm), the column temperature is 20°C, the injection volume is 1mL, and the flow rate is 5mL / min.
[0032] The separated product was analyzed by HPLC under the following conditions: the mobile phase methanol: water: diethylamine volume ratio was 50:50:0.02, the chromatographic column was a cosmosil MS-II C18 column, the column temperature wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com