Hydrophilic processing method for zinc nickle alkaline accumulator diaphragm
A technology for hydrophilic treatment and storage batteries, which is applied in battery pack parts, circuits, electrical components, etc., can solve the problems of inability to apply zinc-nickel storage batteries, and achieve good hydrophilic liquid absorption performance, improved cycle life, and good air permeability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] This embodiment illustrates a method for treating non-hydrophilic diaphragms with hydrophilicity:
[0049] a. Preparation of hydrophilic treatment solution
[0050] By weight percentage as follows:
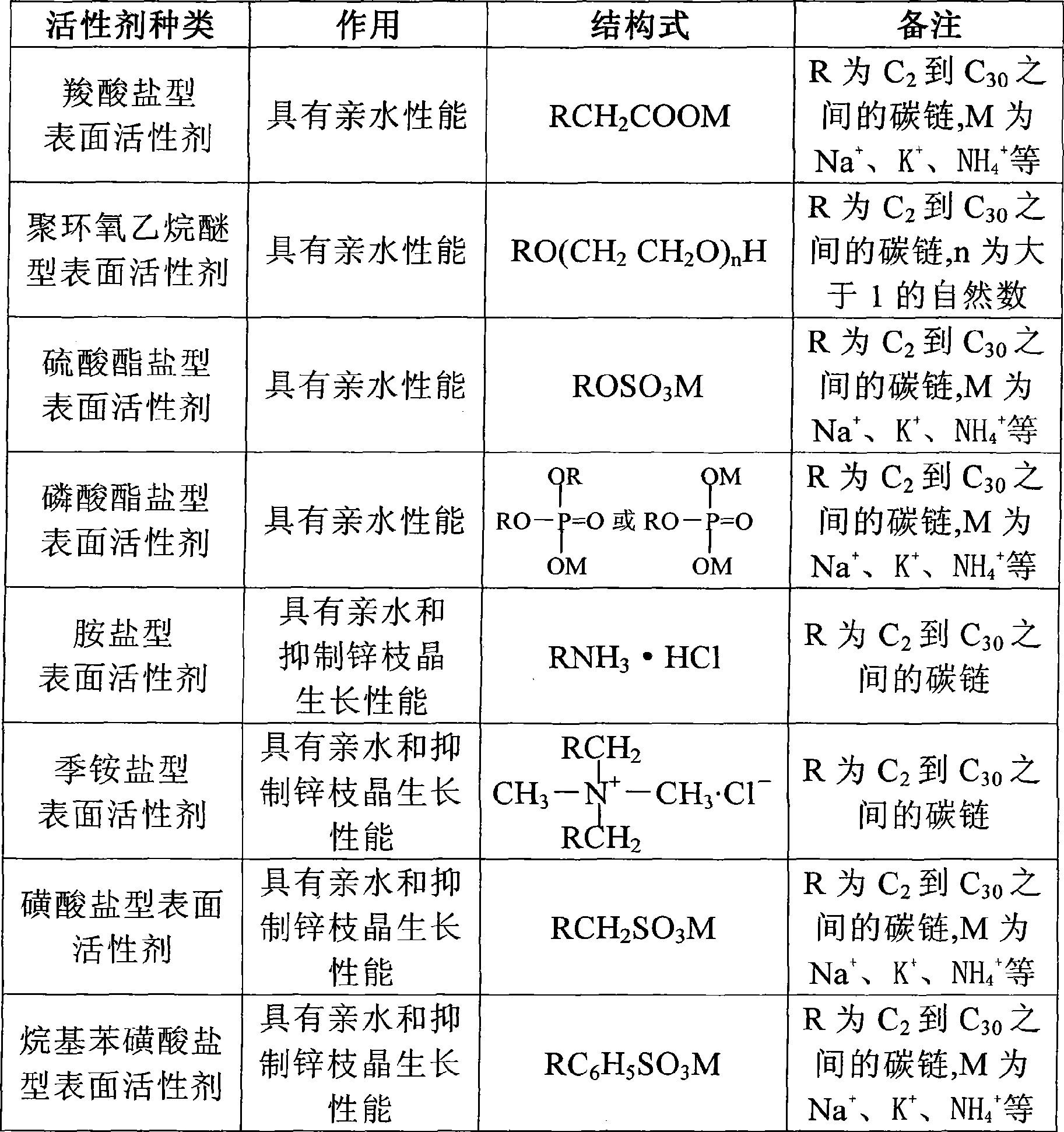
[0051] Hydrophilic Surfactant: C 8 h 17 CH 2 COONa 0.1%
[0052] Oxidant: hydrogen peroxide 1%
[0053] Ketones: Acetone 3%
[0054] Alcohols: Isopropanol 3%
[0055] Deionized water: balance
[0056] b. The non-hydrophilic diaphragm is completely immersed in the hydrophilic treatment solution for 5 minutes;
[0057] c. Clean the treated diaphragm with deionized water until the pH value reaches 7;
[0058] d. Prepare coating solution
[0059] By weight percentage as follows:
[0060] Additives for inhibiting zinc dendrite growth: C 8h 17 CH 2 NH 3 ·HCl 6%
[0061] Deionized water: balance
[0062] e. The diaphragm is completely immersed in the coating layer solution for 2 minutes;
[0063] f. Dry at 65°C or dry naturally.
[0064] Note: This proportion of...
Embodiment 2
[0068] This embodiment illustrates a method for treating non-hydrophilic diaphragms with hydrophilicity:
[0069] a. Preparation of hydrophilic treatment solution
[0070] By weight percentage as follows:
[0071] Hydrophilic Surfactant: C 9 h 19 CH 2 OSO 3 Na 6%
[0072] Oxidizer: sodium hypochlorite 3%
[0073] Ketones: Acetone 2%
[0074] Alcohols: Isopropanol 2%
[0075] Alkalis: Potassium Hydroxide 0.5%
[0076] Deionized water: balance
[0077] b. The non-hydrophilic diaphragm is completely immersed in the hydrophilic treatment solution for 20 minutes;
[0078] c. Clean the treated diaphragm with deionized water until the pH value reaches 7;
[0079] d. Prepare coating solution
[0080] By weight percentage as follows:
[0081] Additives for inhibiting zinc dendrite growth: C 8 h 17 CH 2 NH3·HCl 6%
[0082] Deionized water: balance
[0083] e. The diaphragm is completely immersed in the coating layer solution for 5 minutes;
[0084] f. Dry at 75°C or d...
Embodiment 3
[0087] This embodiment illustrates a method for treating non-hydrophilic diaphragms with hydrophilicity:
[0088] a. Preparation of hydrophilic treatment solution
[0089] By weight percentage as follows:
[0090] Hydrophilic Surfactant: C 12 h 25 C 6 h 5 SO 3 K 16%
[0091] Oxidant: hydrogen peroxide 1%
[0092] Ketones: Acetone 2%
[0093] Alcohols: Ethanol 3%
[0094] Deionized water: balance
[0095] b. The non-hydrophilic diaphragm is completely immersed in the hydrophilic treatment solution for 30 minutes;
[0096] c. Clean the treated diaphragm with deionized water until the pH value reaches 7;
[0097] d. Prepare coating solution
[0098] By weight percentage as follows:
[0099] Additive for inhibiting zinc dendrite growth: tetrabutylammonium bromide 0.1%
[0100] Deionized water: balance
[0101] e. The diaphragm is completely immersed in the coating layer solution for 1min;
[0102] f. Dry at 85°C or dry naturally.
[0103] Note: This proportion of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com