RGD polypeptide radiopharmaceutical for integrin alphav beta3 positive tumor and preparation method thereof
A radiopharmaceutical and radionuclide technology, applied in the field of RGD polypeptide radiopharmaceuticals and their preparation, can solve problems such as insufficient distance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 111
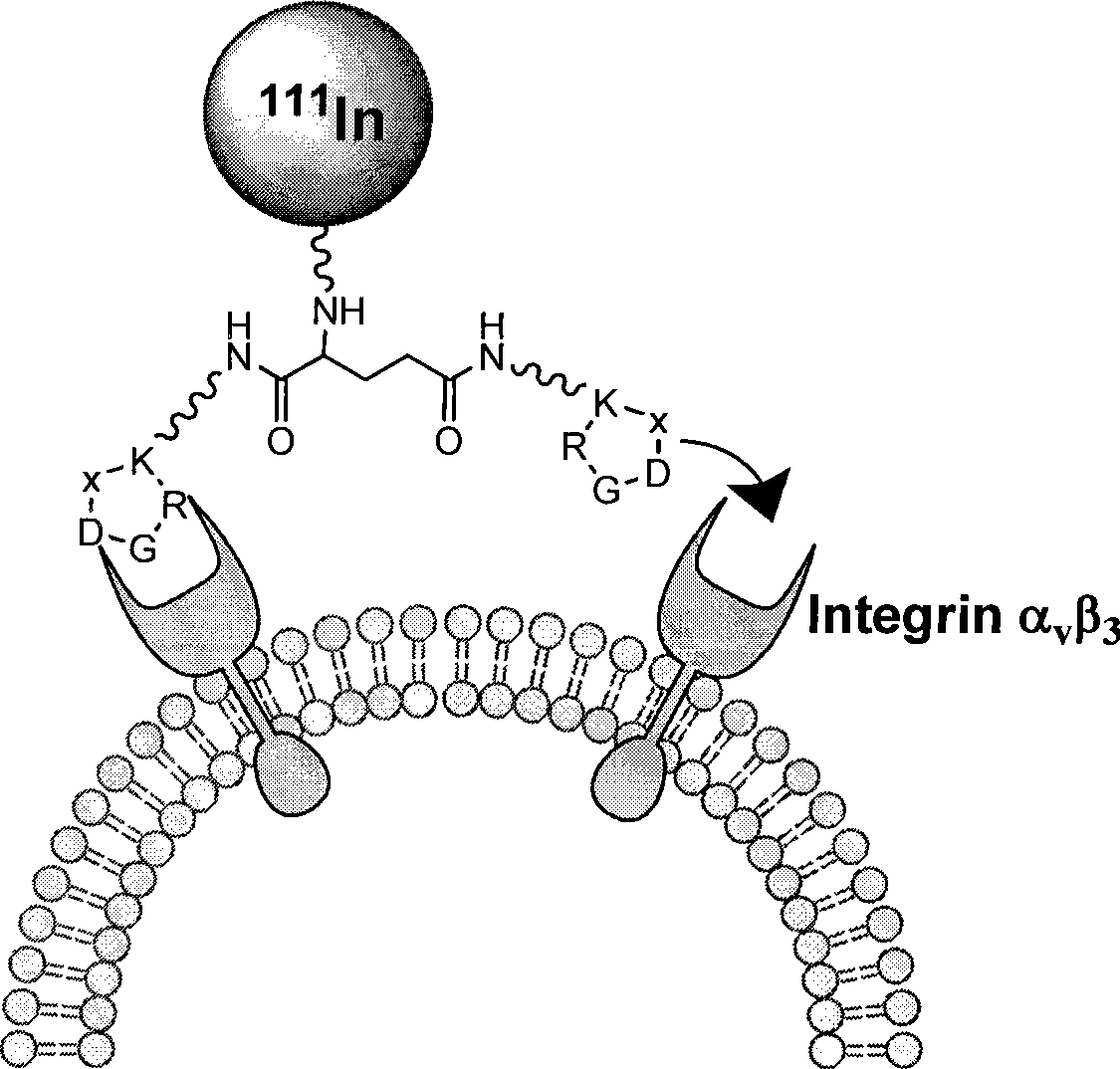
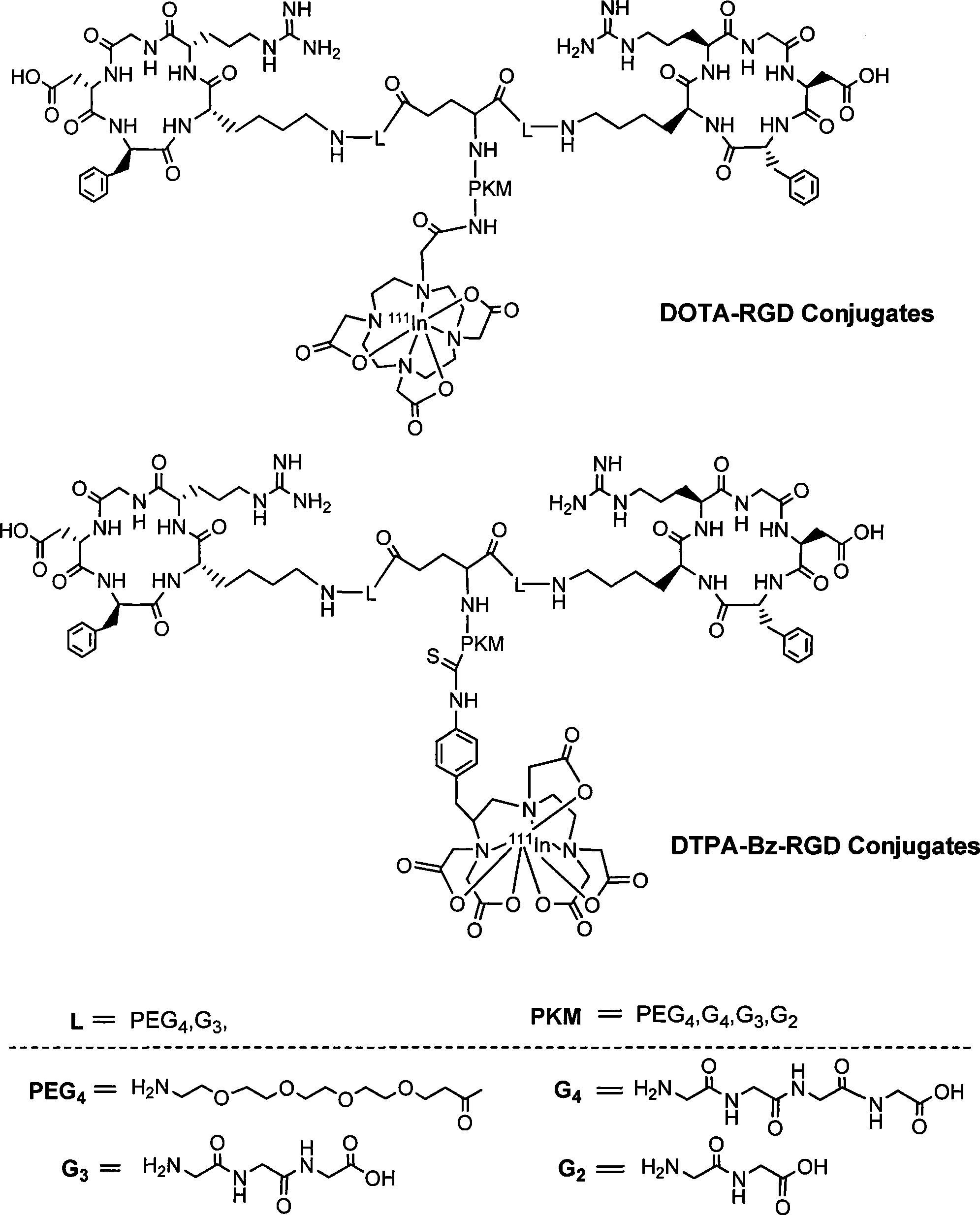
[0036] This embodiment takes 111 In-DOTA-PEG 4 -E[PEG 4 -c(RGDfK)] 2 (referred to as 111 In-DOTA-3PEG 4 -dimer) RGD polypeptide radiopharmaceutical and its preparation method as an example.
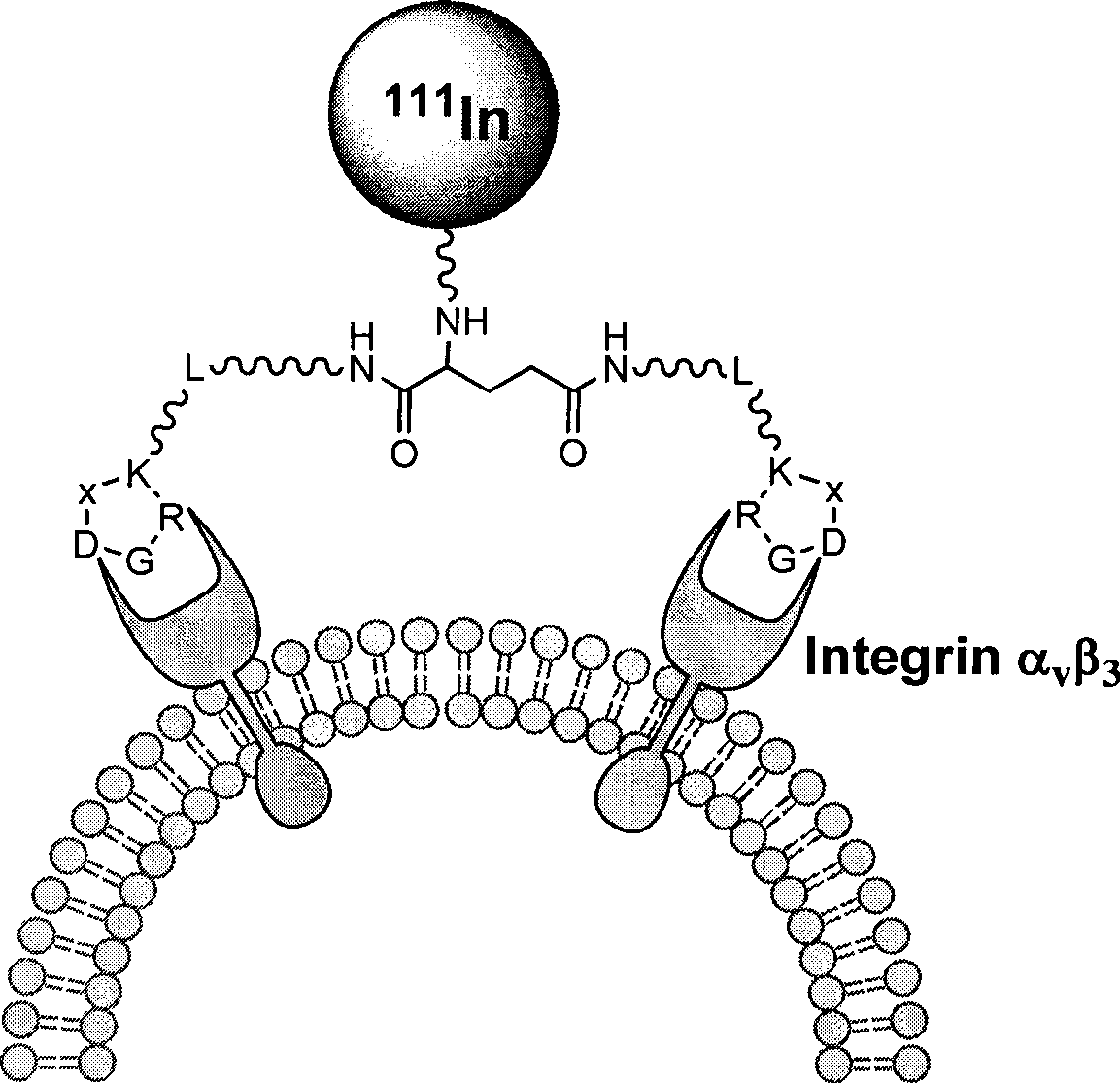
[0037] 111 In-DOTA-PEG 4 -E[PEG 4 -c(RGDfK)] 2 (referred to as 111 In-DOTA-3PEG 4 -dimer) polypeptide radiopharmaceuticals including RGD cyclic peptide dimer, bifunctional chelator DOTA and radionuclide 111 In. The RGD cyclic peptide dimer is the linker PEG 4 Linked with RGD polypeptide monomer c (RGDfK), and then two linked with PEG 4 RGD polypeptide monomer PEG 4 The RGD cyclic peptide dimer synthesized by dimerization of -c(RGDfK), that is, E[PEG 4 -c(RGDfK)] 2 , radionuclide 111 In labels the RGD cyclic peptide dimer with a bifunctional chelating agent DOTA, and a pharmacokinetic modification molecule PEG is also connected between the RGD cyclic peptide dimer and the bifunctional chelating agent 4 , the RGD polypeptide radiopharmaceutical is 111 In-DOTA-PEG 4 -E[PEG ...
Embodiment 2
[0064] In this example, the RGD polypeptide radiopharmaceutical of the present invention uses bifunctional chelating agent DOTA or TETA or its derivatives to label radionuclide 64 Cu, when linker L is PEG 4 、Pharmacokinetic modification molecule PKM is PEG 4 When, the drug of the present invention is 64 Cu-DOTA-PEG4 -E[PEG 4 -c(RGDfK)] 2 ( 64 Cu-DOTA-3PEG 4 -dimer) or 64 Cu-TETA-PEG 4 -E[PEG 4 -c(RGDfK)] 2 ( 64 Cu-TETA-3PEG 4 -dimer), its preparation method is the same as in Example 1. Pharmacokinetic modifier molecules PKM can also be G 2 or G 4 .
[0065] When linker L is G 3 , pharmacokinetic modification molecule PKM is G 3 When, the drug of the present invention is 64 Cu-DOTA-G 3 -E[G 3 -c(RGDfK)] 2 ( 64 Cu-DOTA-3G 3 -dimer) or 64 Cu-TETA-G 3 -E[G 3 -c(RGDfK)] 2 ( 64 Cu-TETA-3G 3 -dimer), its preparation method is the same as above. Pharmacokinetic modifier molecules PKM can also be G 2 or G 4 .
[0066] In this example, the RGD polypeptide...
Embodiment 3
[0068] In this example, the RGD polypeptide radiopharmaceutical of the present invention uses bifunctional chelating agent DOTA or NOTA or its derivatives to label radionuclide 68 Ga. When linker L is PEG 4 、Pharmacokinetic modification molecule PKM is PEG 4 When, the drug of the present invention is 68 Ga-DOTA-PEG 4 -E[PEG 4 -c(RGDfK)] 2 ( 68 Ga-DOTA-3PEG 4 -dimer) or 68 Ga-NOTA-PEG 4 -E[PEG 4 -c(RGDfK)] 2 ( 68 Ga-NOTA-3PEG 4 -dimer), its preparation method is the same as in Example 1. Pharmacokinetic modifier molecules PKM can also be G 2 or G 4 .
[0069] When linker L is G 3 , pharmacokinetic modification molecule PKM is G 3 When, the drug of the present invention is 68 Ga-DOTA-G 3 -E[G 3 -c(RGDfK)] 2 ( 68 Ga-DOTA-3G 3 -dimer) or 68 Ga-NOTA-G 3 -E[G 3 -c(RGDfK)] 2 ( 68 Ga-NOTA-3G 3 -dimer). Pharmacokinetic modifier molecules PKM can also be G 2 or G 4 .
[0070] In this example, the RGD polypeptide radiopharmaceutical of the present invent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com