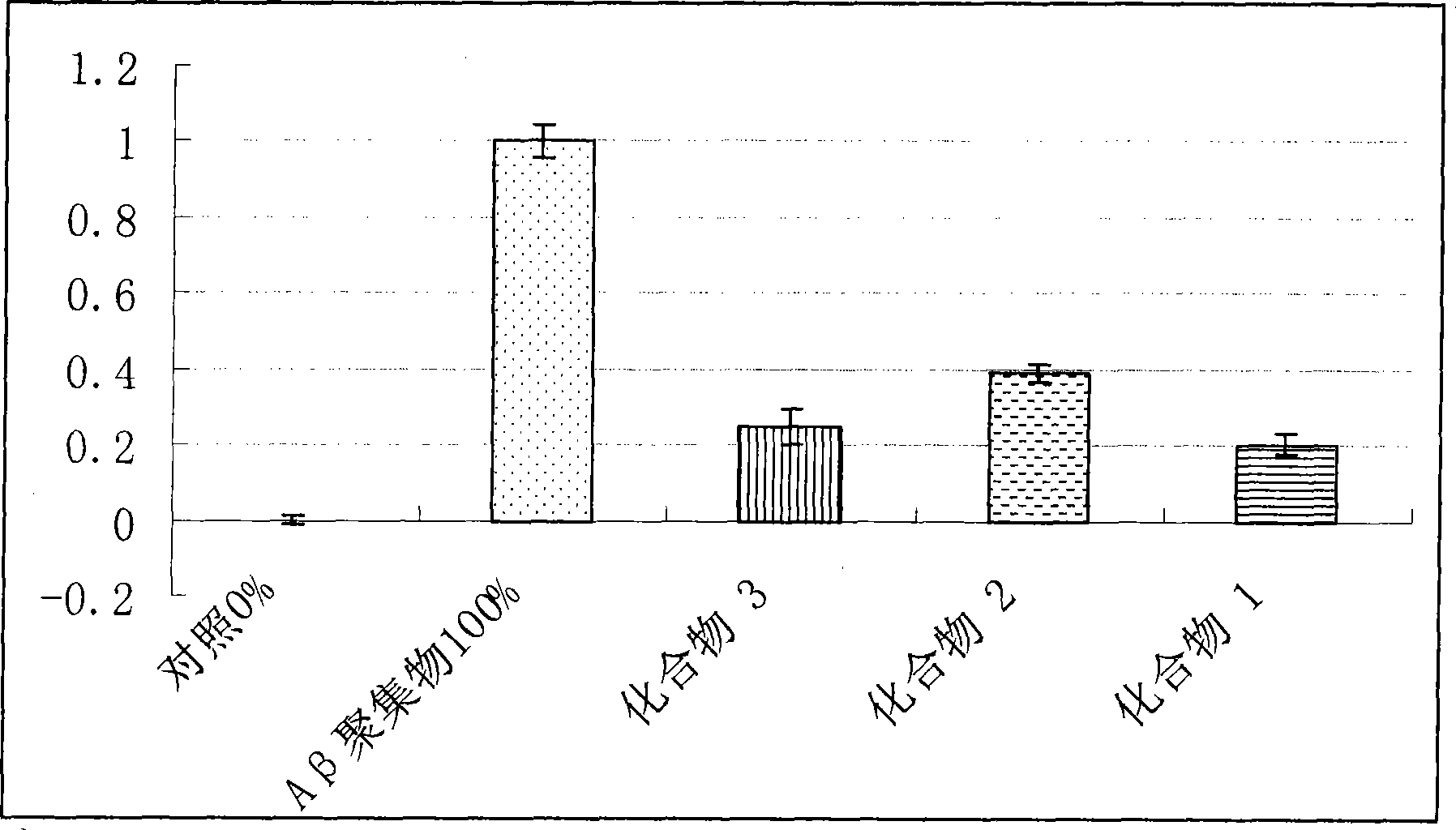Medical use of beta-(3,4-dihydroxy phenyl)-alpha-hydroxy propionic ester compounds
A technology of polyphenolic compounds and compounds, which is applied in the field of β-α-hydroxypropionate compounds, can solve the problems of salvia miltiorrhiza compounds, and achieve the effects of preventing Aβ aggregation, promoting the proliferation of neural stem cells, and promoting the degradation of Aβ
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0064] Compound Preparation Example 1: Preparation of β-(3,4-Dihydroxyphenyl)-α-Hydroxybornyl Propionate (Compound 1)
[0065] 0.12mol of β-(3,4-dihydroxyphenyl)-α-hydroxypropionic acid (extracted in this laboratory, purity 96%) and 0.18mol of borneol were added to 500ml of tetrahydrofuran, and the catalyst p-toluenesulfonic acid 0.86 g, reacted at 65°C for 8h. After the reaction, the catalyst, solvent and unreacted borneol were removed to obtain a brown viscous substance, which was separated by column chromatography to obtain a pale yellow oil (compound 1).
[0066] IR v(liquid paraffin)cm -1 : 3320, 2955, 2915, 1810, 1596, 1519, 1450, 1280, 1113, 885, 801.
[0067] ESI+: 335
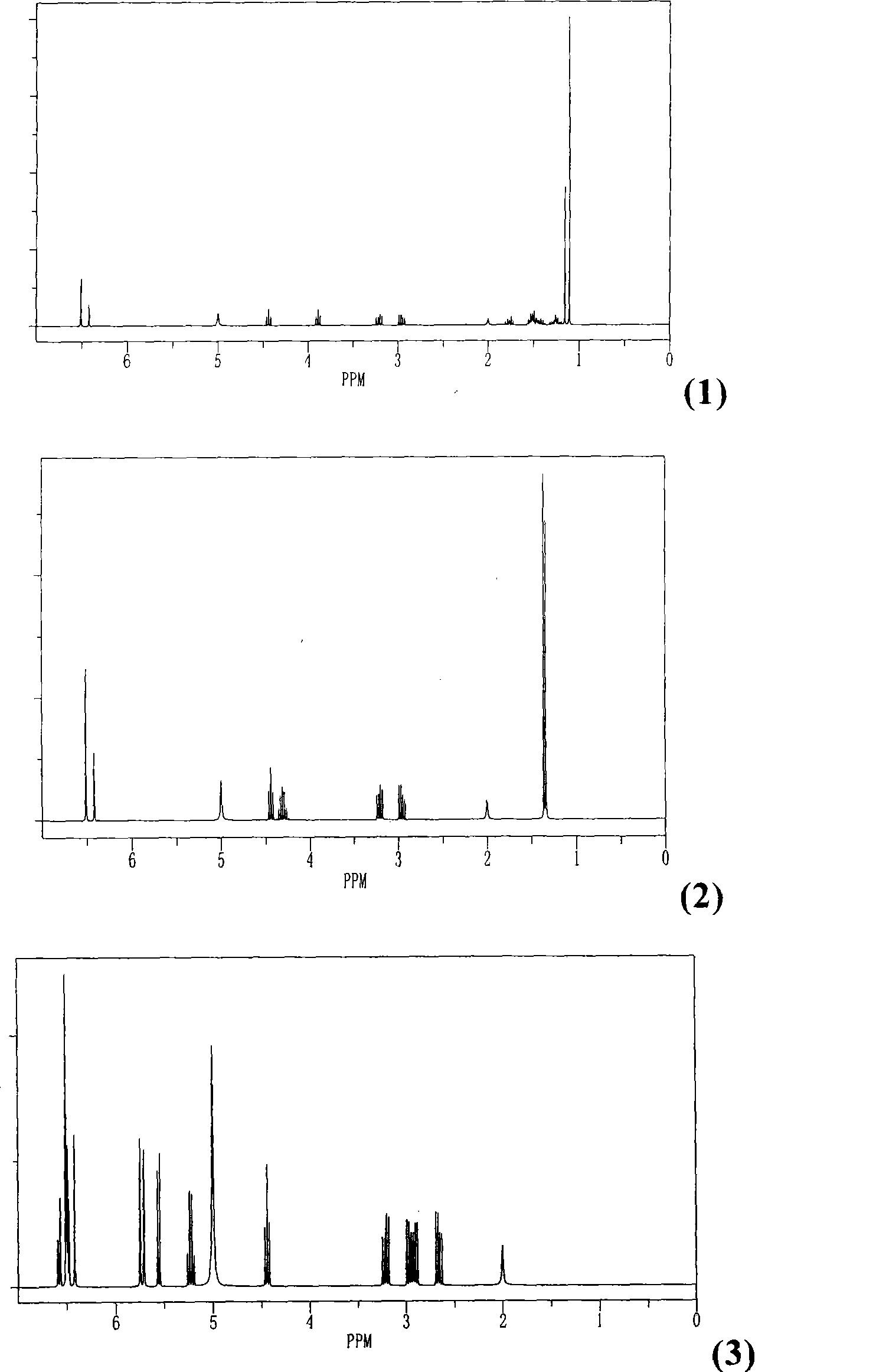
[0068] 1 H NMR see figure 1 (1).
preparation example 2
[0069] Compound Preparation Example 2: Preparation of β-(3,4-dihydroxyphenyl)-α-hydroxypropionic acid isopropyl ester (compound 2)
[0070]
[0071] Compound 2
[0072] According to the above reaction process, 0.1 mol of compound a and 0.1 mol of compound b were dissolved in water, reacted at room temperature for 4 hours, and c was isolated. c was reacted with sodium acetate and 3,4-dihydroxybenzaldehyde according to 1:1.2:1.5 at 100°C for 30min, and d was separated. d first reacted with zinc-amalgam concentrated hydrochloric acid for 7 hours under reflux, then added sodium bicarbonate to adjust the pH value to about 5, and e was precipitated. e reacts with isopropanol at room temperature according to 1:1.2 to generate the target product compound 2.
[0073] ESI+: 241
[0074] 1 H NMR see figure 1 (2).
preparation example 3
[0075] Compound Preparation Example 3: Preparation of β-(3,4-dihydroxyphenyl)-α-hydroxypropionate catechin prepare (compound 3)
[0076] According to conventional ester-forming method, select relatively mild condition, 0.1mol salvia miltiorrhiza (extracted in this laboratory, purity 96.8%) and 0.18mol catechin are dissolved in 200ml benzene, add catalyst p-toluenesulfonic acid 0.86g, Reaction at room temperature for 12h. After the reaction, the catalyst, solvent and unreacted catechin were removed to obtain compound 3.
[0077] ESI+: 471
[0078] 1 H NMR see figure 1 (3).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com