Process for preparing dimethyl ether
A technology of dimethyl ether and methanol, applied in ether preparation, ester reaction to prepare ether, organic chemistry, etc., can solve the problems of high by-product sulfuric acid, large consumption of raw materials, etc., and achieve high utilization rate of raw materials, low equipment requirements, and energy consumption. reduced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
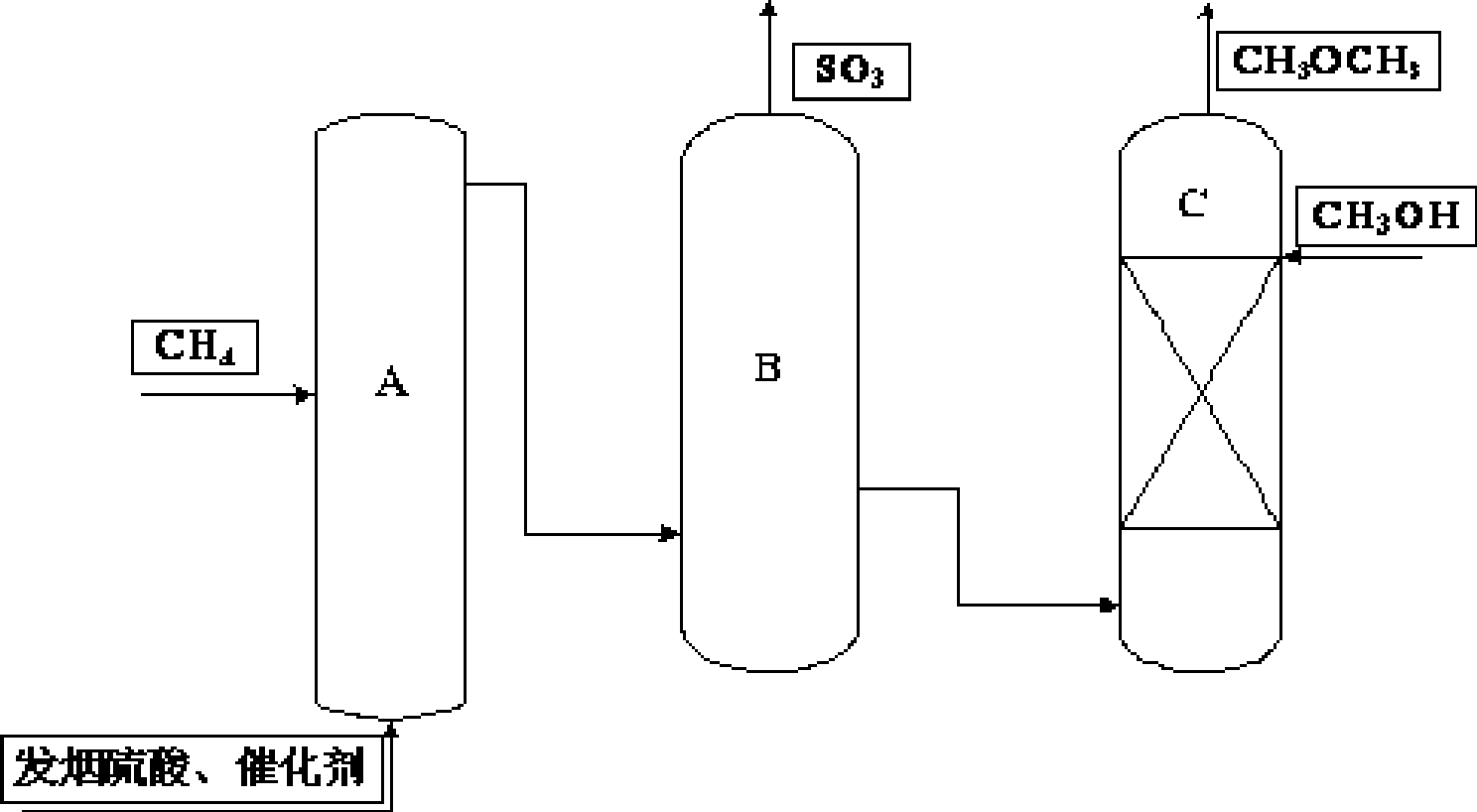
[0041] Add dimethyl sulfate and methanol to the stainless steel alcoholysis kettle C. The molar ratio of dimethyl sulfate to methanol is 1:2. The alcoholysis is carried out at 35°C for 1 hour. The vaporized gas is collected by condensation and drying, and then subjected to gas chromatography. Analysis shows that the gas phase composition contains 98% dimethyl ether; after quantitative analysis, the conversion rate of dimethyl sulfate is 82% and the selectivity is 100%.
Embodiment 2
[0043] Pass the methane into the reactor A, and at the same time pass the dissolved catalyst I 2 Contains fuming sulfuric acid, which contains 30% SO 3 , Catalyst I 2 The concentration of is 0.0014mol / L, and the reaction is performed at a temperature of 200℃ and a pressure of 0.1MPa; then the mixture containing methyl hydrogen sulfate obtained after the reaction is introduced into SO 3 In distillation tower B, distill at 35℃ for 72 hours to remove SO 3 ; After the distillation, the mixture containing dimethyl sulfate and methyl hydrogen sulfate is introduced into the alcoholysis kettle C, and methanol is slowly added. The molar ratio of sulfuric esters to methanol is 1:1.2, and the temperature is 180°C. After 1.5h of alcoholysis, the vaporized gas is collected by condensation and drying, and analyzed by gas chromatography. The gas phase composition contains 99% of dimethyl ether; after quantitative analysis, the conversion rate of sulfate esters is 100%, and the selectivity is 100...
Embodiment 3
[0045] Pass the methane into the reactor A, and at the same time pass the fuming sulfuric acid with the catalyst sodium iodide and potassium iodide, the fuming sulfuric acid contains 40% SO 3 , The total concentration of the catalyst sodium iodide and potassium iodide is 0.011mol / L, and the reaction is performed at a temperature of 150°C and a pressure of 15MPa; then the mixture containing methyl hydrogen sulfate obtained after the reaction is introduced into SO 3 In distillation tower B, distill at 200℃ for 10 hours to remove SO 3 ; The mixture containing dimethyl sulfate and methyl hydrogen sulfate obtained after distillation is introduced into the alcoholysis kettle C, and methanol is slowly added. The molar ratio of sulfate esters to methanol is 1:1.8, and the temperature is 50 ℃ After alcoholysis for 2h, the vaporized gas is collected by condensation and drying, and analyzed by gas chromatography. The gas phase composition contains 98% of dimethyl ether; after quantitative an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com