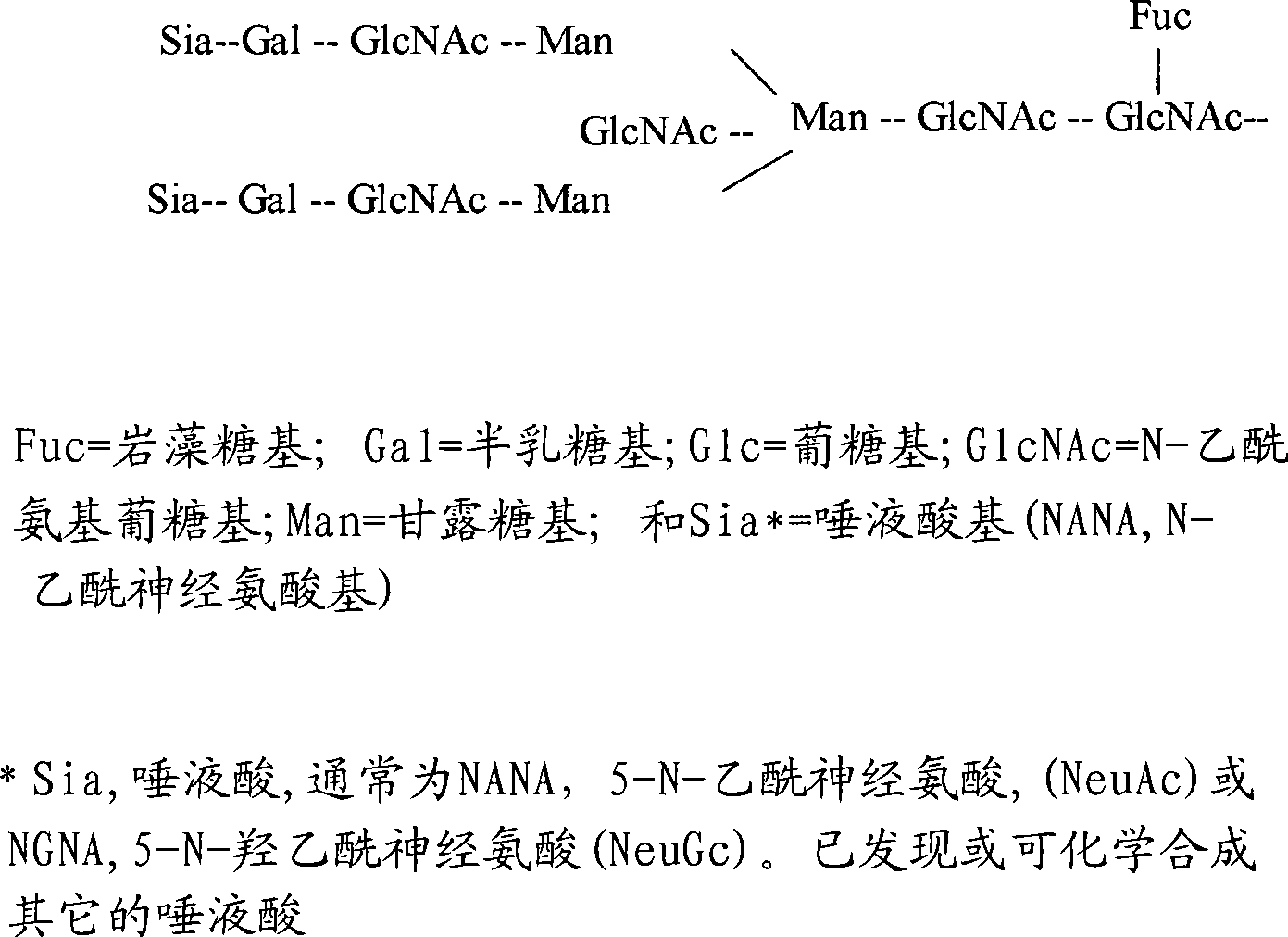
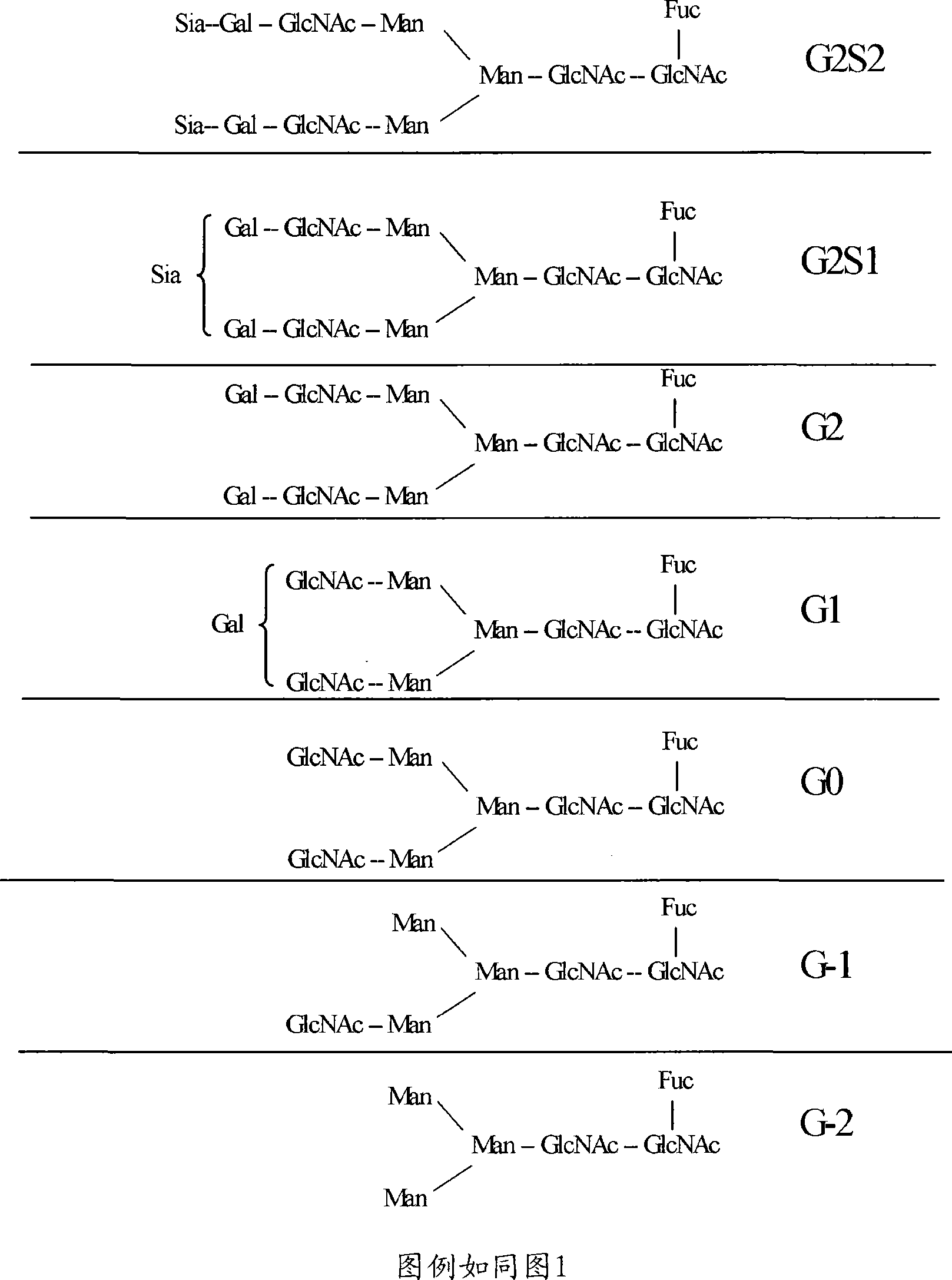
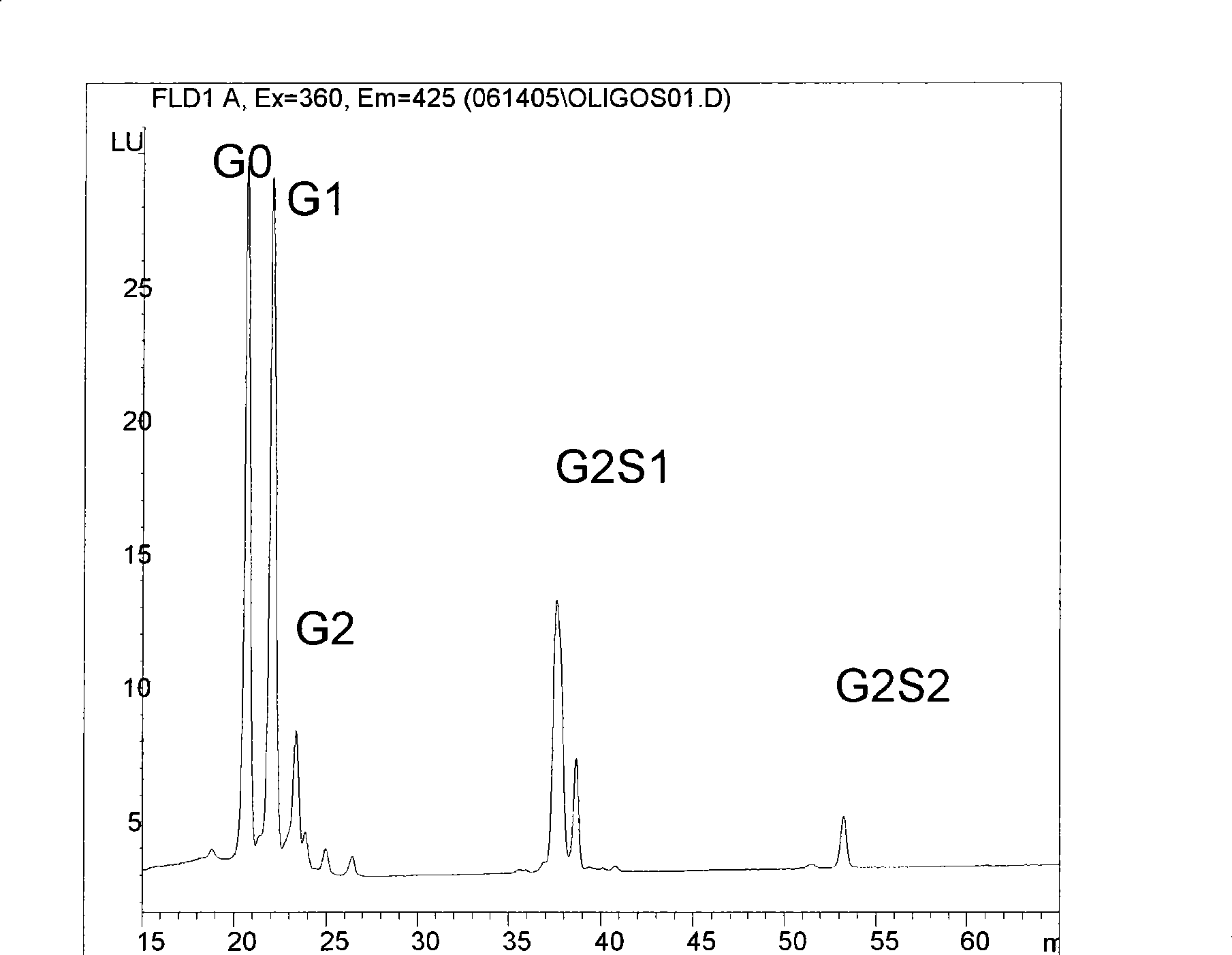
Methods and compositions with enhanced therapeutic activity
A molecular and property technology, applied in the field of improving therapeutic activity and components, can solve problems such as lack of Fc function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Example 1: Lectin-based separation of antibody species with different levels of sialic acid
[0118] Agarose beads coupled with MAA (Huaihua Agglutinin) or WGA (Wheat Germ Agglutinin) were purchased from Vector Labs or EY Labs. The test antibody Ab1 is a fully human monoclonal antibody that binds human TNF. will contain 20mM CaCl 2 and 20mM MgCl 2 Ab1 (~1-10 mg) in 20 mM Tris-HCl buffer (pH 7.0) was fractionated on MAA-Sepharose or WGA-Sepharose columns. Ab1 bound to the MAA-Sepharose column was eluted with 0.5% acetic acid, neutralized with 1M Tris-HCl (pH 7.0), and then the buffer was exchanged with PBS. This substance is called Ab1 MAAB.
[0119] After loading Ab1 antibody samples onto a WGA-Sepharose column, the non-bound (T, passed) fraction was collected and the buffer was exchanged for PBS. This substance is called Ab1WGAT. The column containing bound Ab1 was washed with the same buffer as above, and a 1 ml fraction of any eluted antibody (representing weak...
Embodiment 2
[0121] Example 2: Enzyme Modification of Galactosylated and Sialylated Antibodies
[0122] To enzymatically galactosylate purified antibody samples, bovine β-1,4-galactosyltransferase (β1,4GT) from Sigma Chemical Co. (St. Louis, MO) and UDP-Gal. Recombinant rat liver α-2,3-sialyltransferase (α2,3ST), recombinant α-1,3-galactosyltransferase (α1,3GT) and CMP-Sia were from Calbiochem (San Diego, CA ). PNGase F was from New England Biolabs (Beverly, MA) or from Prozyme (San Leandro, CA) or from Selectin BioSciences (Pleasant Hill, CA). β-galactosidase and β-glucosaminidase of Diplococcus pneumoniae were obtained from ProZyme or Selectin BioSciences. Beta-galactosidase from bovine kidney and all other enzymes were obtained from ProZyme or from Selectin BioSciences. NAP-5 and HiTrap protein A columns were obtained from Pharmacia Biotech (Piscataway, NJ). All other reagents were of analytical grade.
[0123] An enzymatically deglycosylated form of Abl (termed Gno) was prepared ...
Embodiment 3
[0135] Example 3: Binding to low affinity cellular FC receptors
[0136] Of the several types of Fc receptors on effector cells, Fcγ types II and III are considered low or intermediate affinity receptors. Typically, the binding of the monomer may be with too low affinity to be detected or at a very low level. For example, binding of monomeric IgG to gamma IIA is difficult to measure. These receptors act to bind immune complexes due to their more avid, multivalent nature of binding, perhaps due to the slowed rate of the complexes.
[0137] Human K562 cells, which express FcγRIIA as the sole Fcγ receptor, were used in two types of binding assays to test whether alterations in the acid saliva content of Fc glycans affect binding to this low-affinity human Fcγ receptor . To obtain binding with sufficient affinity to FcγRIIA (which has low affinity for monomeric IgG), an immune complex was prepared by mixing the test Ab against TNF with homotrimeric TNF in a 2:1 molar ratio , t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com