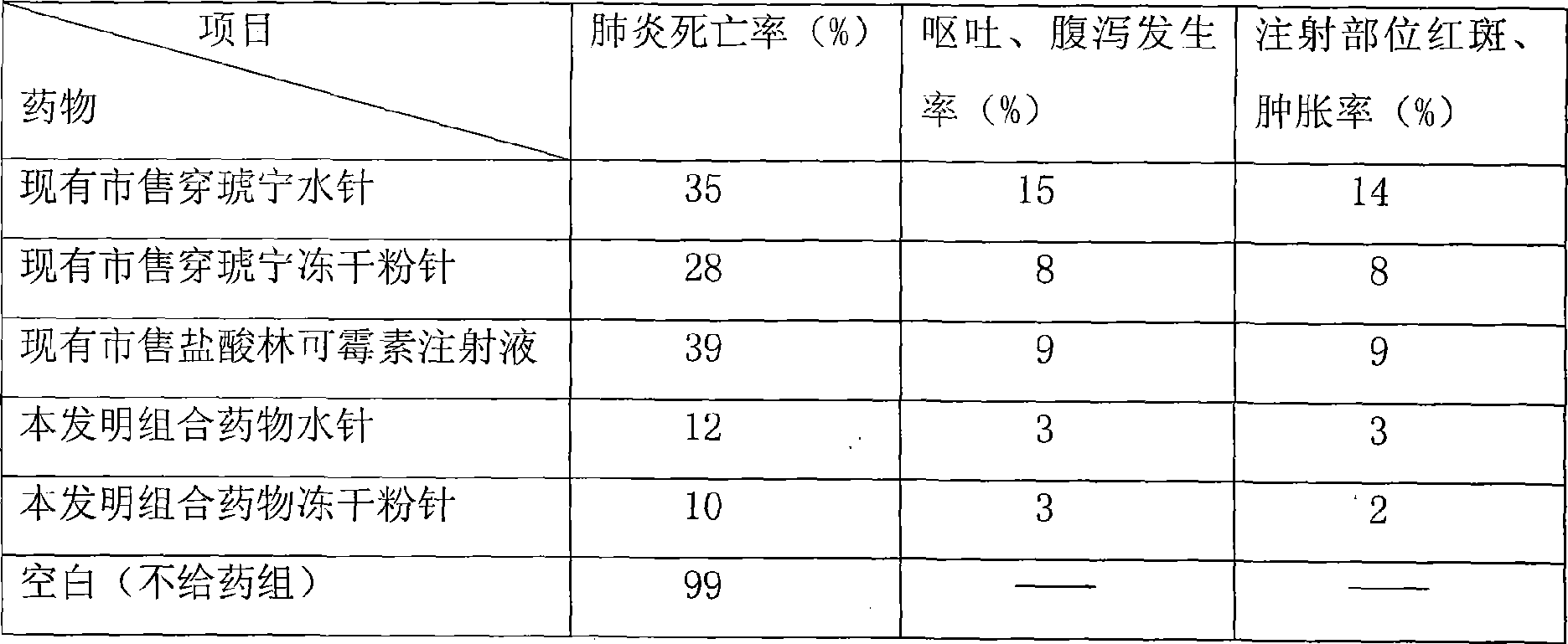
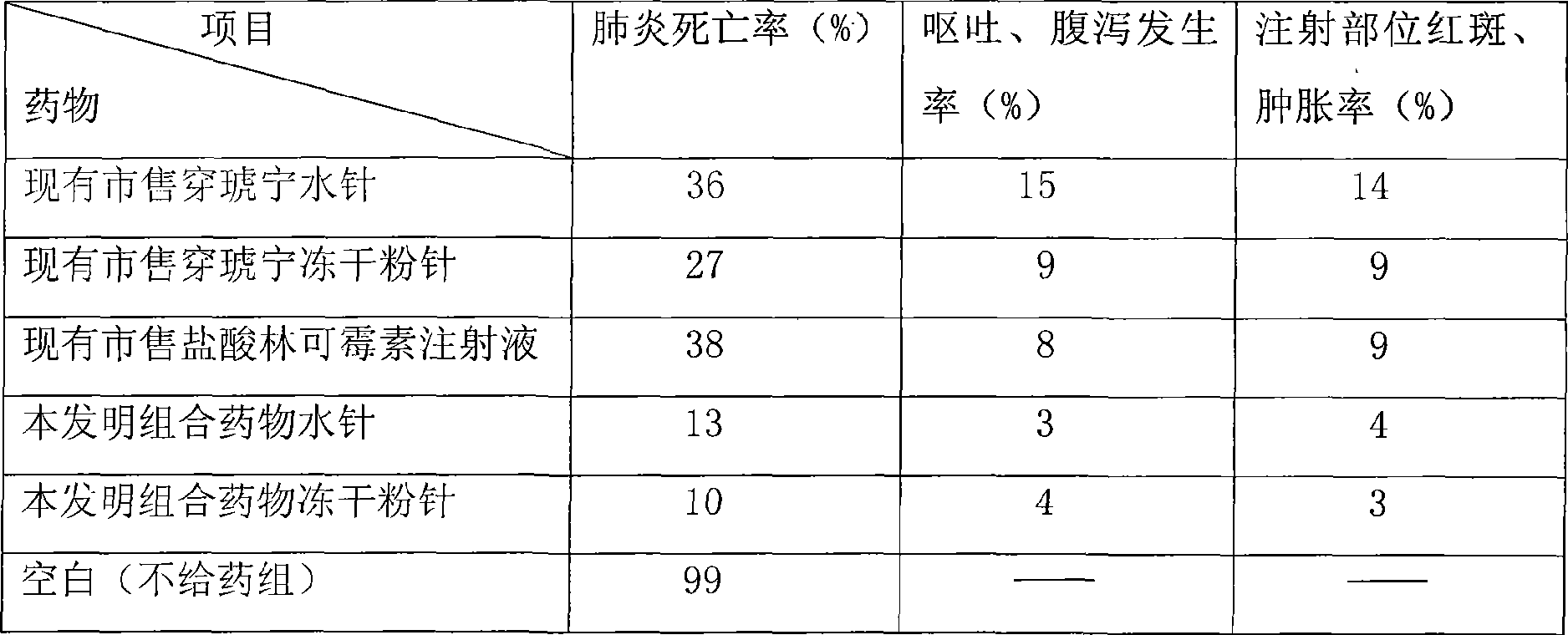
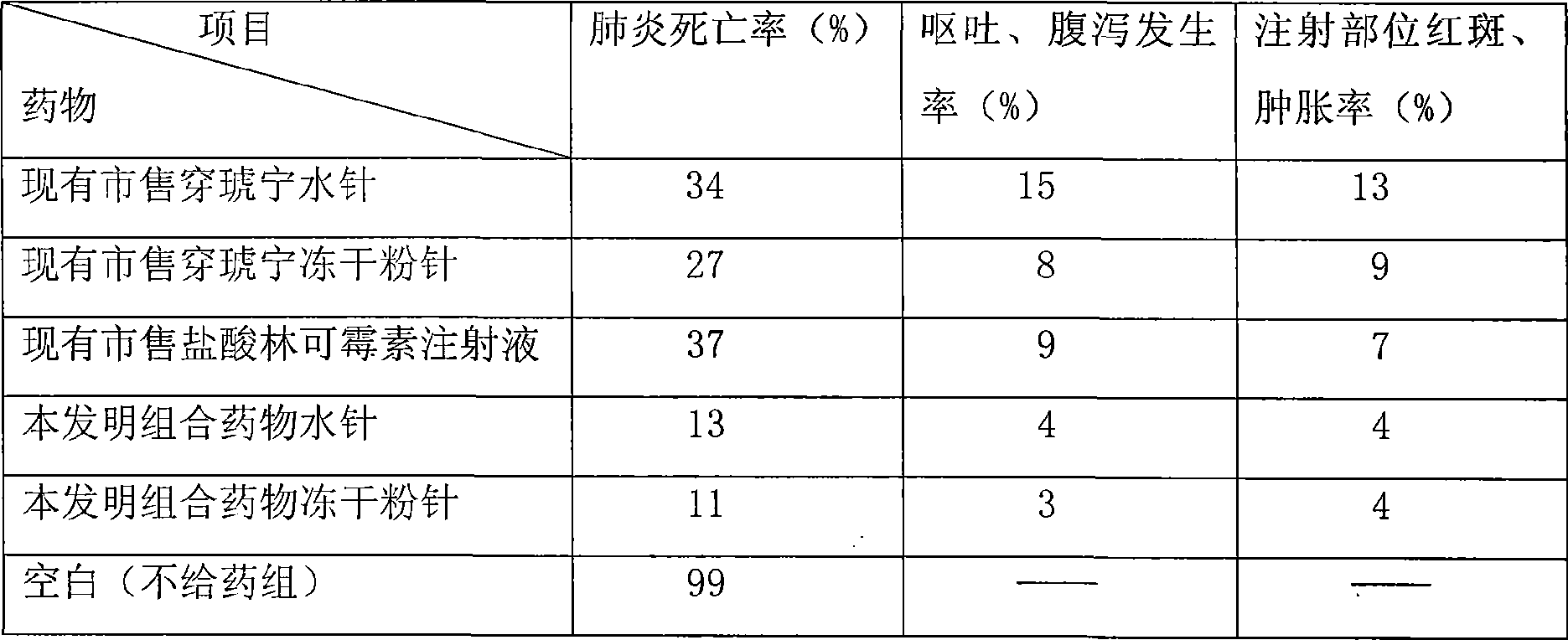
Combination medicine capable of treating pneumonia and upper respiratory tract infection and preparation method thereof
A technology for the upper respiratory tract and drugs, applied in drug combinations, pharmaceutical formulations, respiratory diseases, etc., can solve the problems of simple dosage forms and difficult to eliminate adverse reactions, and achieve low cost, high production efficiency, and good economical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] The preparation method of the combination medicine of the present invention, its specific steps are as follows:
[0020] 1. After dissolving the EDTA-Ca in the weight ratio and adding water for injection, add the sodium thioglycolate in the weight ratio, and add disodium hydrogen phosphate or sodium dihydrogen phosphate (disodium hydrogen phosphate or phosphoric acid) after dissolving until clarified. The amount of sodium dihydrogen to be added is based on adjusting the pH value to 6.0 to 7.0) to adjust the pH value to 6.0 to 7.0 to obtain No. 1 reserve solution;
[0021] 2. Dissolve the disodium hydrogen phosphate in the weight ratio by adding water for injection, measure the pH value to 9.0-10.0, control the temperature at 42°C-48°C, stir continuously under nitrogen, slowly add the Chuanhuning in the weight ratio, Make it completely dissolved until it becomes clear, and the measured pH value is 6.0 to 7.0 to obtain the No. 2 reserve solution;
[0022] 3. Dissolve the...
Embodiment 1
[0028] The combined medicine prepared by the present invention is prepared by adding chuanhuning 220g, lincomycin hydrochloride 800g, disodium hydrogen phosphate 30g, sodium thioglycolate 20g, EDTA-Ca 1.0g to 11L with water for injection.
[0029] The preparation method of the combination medicine of the present invention, its specific steps are as follows:
[0030] 1. Take 1.0g of EDTA-Ca and add 1L of water for injection to dissolve, then add 20g of sodium thioglycolate, dissolve until clarified, then add additional disodium hydrogen phosphate or sodium dihydrogen phosphate (what is the amount of disodium hydrogen phosphate or sodium dihydrogen phosphate added? Adjust the pH value to 6.0-7.0 to obtain the No. 1 reserve solution;
[0031] 2. Take 30g of disodium hydrogen phosphate and dissolve it with water for injection, measure the pH value to 9.0-10.0, control the temperature at 42°C-48°C, keep stirring under nitrogen, slowly add 220g of Chuanhuning to make it completely d...
Embodiment 2
[0041] The combined medicine prepared by the present invention can also be prepared by adding 250 g of Chuanhuning, 900 g of lincomycin hydrochloride, 40 g of disodium hydrogen phosphate, 25 g of sodium thioglycolate, and 1.2 g of EDTA-Ca, and adding water for injection to 12 L.
[0042] The preparation method of the combination medicine of the present invention, its specific steps are as follows:
[0043] 1. Take 1.2g of EDTA-Ca and add 1.2L of water for injection to dissolve, then add 25g of sodium thioglycolate, dissolve until clarified, then add additional disodium hydrogen phosphate or sodium dihydrogen phosphate (addition amount of disodium hydrogen phosphate or sodium dihydrogen phosphate How much is subject to adjusting the pH value to 6.0-7.0) Adjust the pH value to 6.0-7.0 to get No. 1 reserve solution;
[0044] 2. Take 40g of disodium hydrogen phosphate and dissolve it with water for injection, measure the pH value to 9.0-10.0, control the temperature at 42°C-48°C, ke...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com