Riemerlla anatipestifer bivalent inactivated vaccine and preparation method thereof
A technology of Ribella and inactivated vaccines, applied in the direction of antibacterial drugs, bacterial antigen components, etc., can solve problems such as difficult to remove, irrational drug regimens, high morbidity and mortality, and achieve saving production and injection costs, health Provides stable development and reduces stress response effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The preparation of embodiment 1 R. anatipestifer inactivated vaccine
[0022] 1. Seed Preparation for Production
[0023] (1) Preparation of first-class seeds: Dissolve the freeze-dried strains of R. anatipestifer in LB medium respectively, inoculate them on tryptone soy agar (TSA) plates containing 2% fetal bovine serum, and place candles Incubate at 37°C for 24 hours in a cylinder. Observe the smooth surface of the bacterial lawn with the naked eye, and select 5 to 10 colonies that are round, slightly protruding, creamy, and slightly blue and translucent;
[0024] The selected colonies were mixed and inoculated with TSA slant containing 2% fetal bovine serum, CO 2 After culturing in an incubator at 37°C for 24 hours, take them out, and observe the pure ones as primary seeds. The primary seeds are stored at 2-8°C.
[0025] (2) Propagation and inspection of secondary seeds: Take primary seeds, wash the bacterial lawn with about 5mL of LB, inoculate in 400mL of LB li...
Embodiment 2
[0036] The preparation of embodiment 2 R. anatipestifer inactivated vaccines
[0037] 1. Seed preparation for production is the same as in Example 1;
[0038] 2. Preparation of bacterial solution for seedling production
[0039] Culture medium is prepared with embodiment 1;
[0040] (1) Bacterial liquid culture: Use a culture tank for ventilated culture, load 70% of the medium and peanut oil defoamer according to the volume, sterilize at 116°C for 40 minutes, and then inoculate the secondary seed liquid according to 5% of the total amount of the medium to gradually increase Large ventilation method, culture at 37°C for 22 hours, type I and type II respectively;
[0041] (2) Pure inspection: After the bacterial culture is completed, take samples for pure inspection;
[0042] (3) Viable bacteria count: Measure the OD525 value, dilute the bacterial solution to an OD525 value of 1, and multiply the dilution factor by 5×10 9 CFU counts viable bacteria, and the counting results ...
Embodiment 3
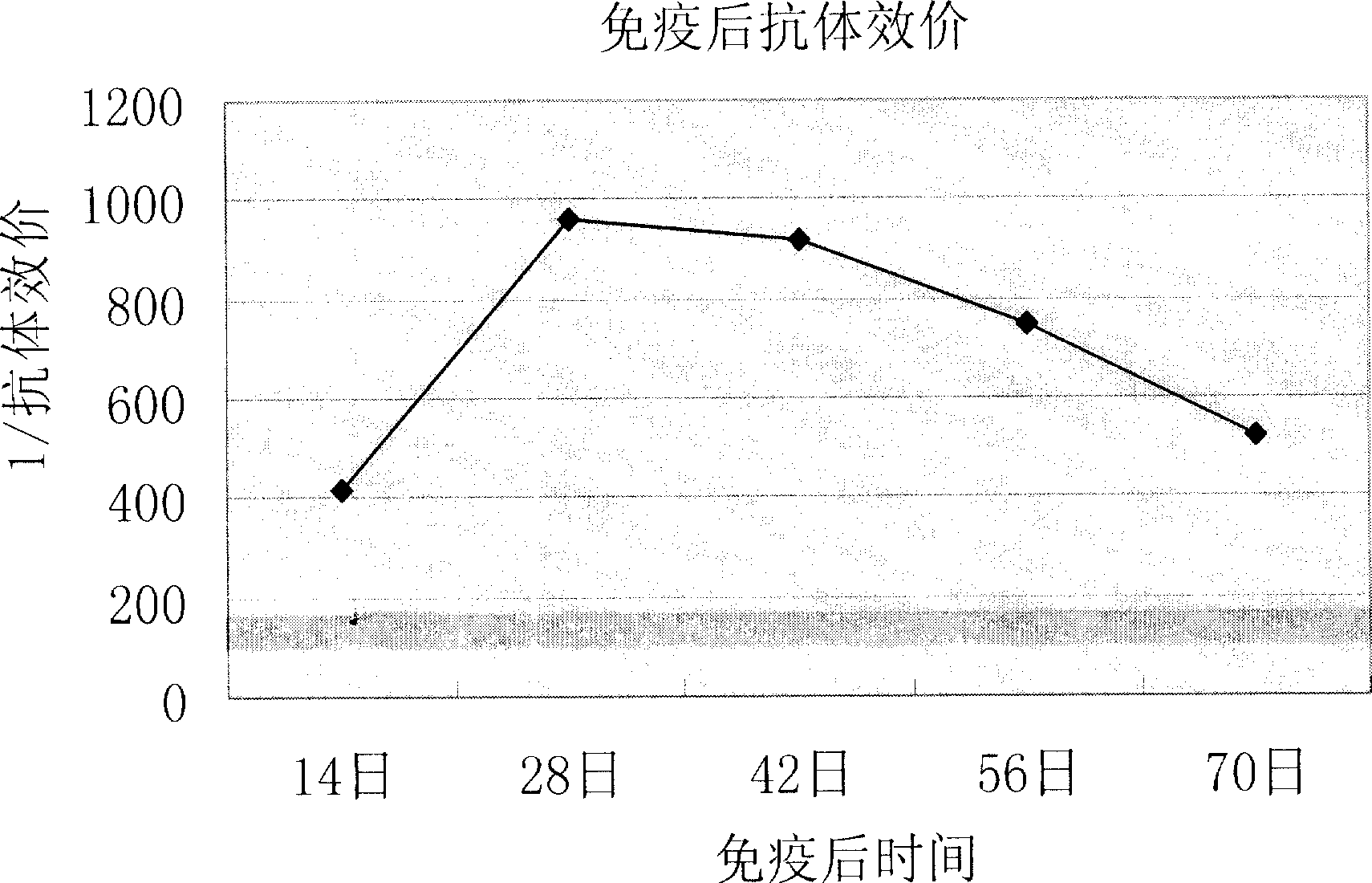
[0048] Embodiment 3 Ribella anatipestifer inactivated vaccine effectiveness test
[0049](1) Antivirus protection test
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com