Process for synthesis of methylation-beta-cyclodextrin with dimethyl carbonate and beta-cyclodextrin
A technology of dimethyl carbonate and cyclodextrin, applied in the field of synthesizing methylated-β-cyclodextrin from dimethyl carbonate and β-cyclodextrin, can solve the problem of increasing risks and restricting methylation-β-cyclodextrin dextrin application and other issues, to achieve the effects of environmental friendliness, high average substitution degree and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
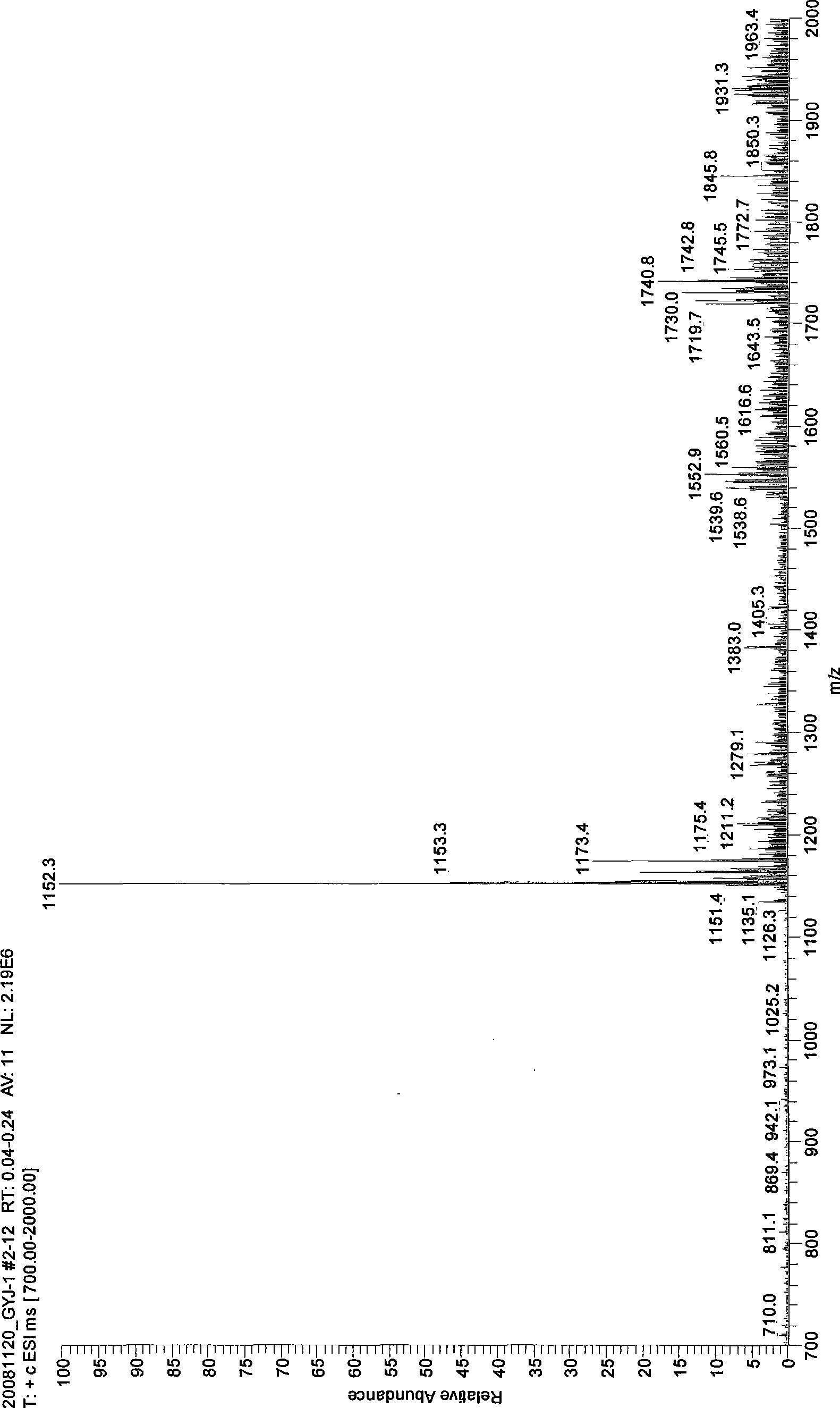
[0018] Embodiment 1: In the three-necked round-bottom flask with reflux condenser and stirring, add 10g β-cyclodextrin (8.8mmol) and stir in 57g dimethylformamide DMF until clarification, then add 8.6g K 2 CO 3 After stirring for 10 minutes, add 3 mL (34 mmol) of dimethyl carbonate, continue to stir, and control the temperature in an ice-water bath to 0° C., and react for 2 hours. The reaction process is monitored by thin-layer chromatography (TLC). After the reaction, the potassium carbonate K was removed by centrifugation. 2 CO 3 and suspended solids, the pressure is 150-260Pa, the liquid phase temperature is 60-85°C, the solvent dimethylformamide DMF and unreacted dimethyl carbonate are distilled off under reduced pressure, the solution is concentrated to syrup, and added to it after cooling 100mL of acetone was stirred, and the acetone was filtered off, and the solid powder obtained was soaked in anhydrous ether for 16 hours, soaked three times to obtain 4.52 grams of th...
Embodiment 2
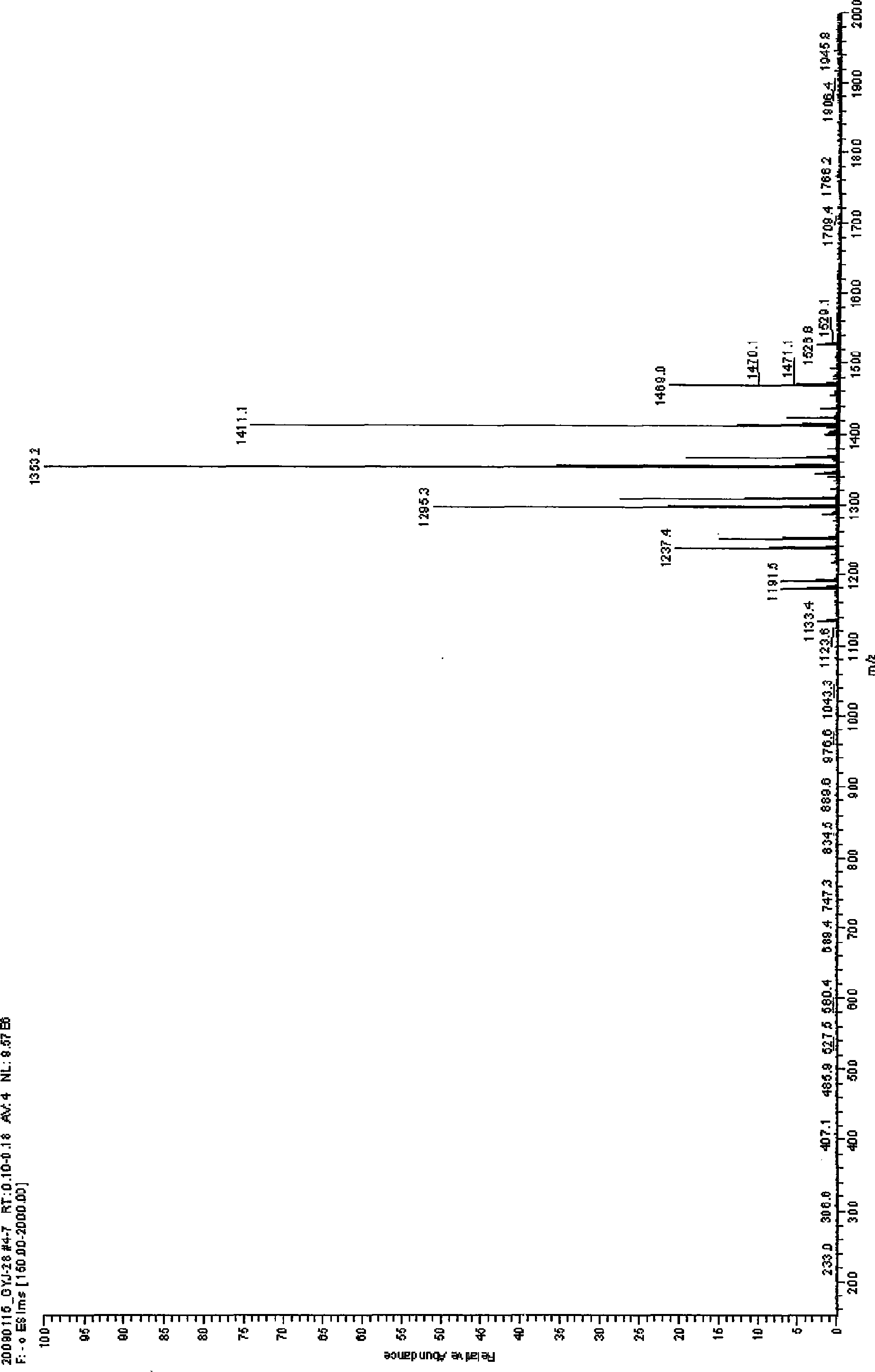
[0019] Embodiment 2: In the three-necked round-bottomed flask with reflux condenser and stirring, add 5gβ-cyclodextrin (4.4mmol) and 57g dimethylformamide DMF and stir until clarification, then add 4.0g potassium carbonate K 2 CO 3 After stirring for 10 minutes, add 5 mL (56.7 mmol) of dimethyl carbonate, continue to stir, heat in a water bath to control the temperature at 50° C., and react for 12 hours. The reaction process is monitored by TLC. After the reaction, K was removed by centrifugation 2 CO 3 and suspended solids, the pressure is 150-260Pa, the liquid phase temperature is 60-85°C, the solvent DMF and unreacted dimethyl carbonate are distilled off under reduced pressure, the solution is concentrated to syrup, after cooling, add 100mL acetone to it, stir, The acetone was filtered off, and the obtained solid powder was soaked in anhydrous ether for 24 hours, soaked twice to obtain 3.45 g of the product, the yield of β-cyclodextrin was 69%, and the average degree of s...
Embodiment 3
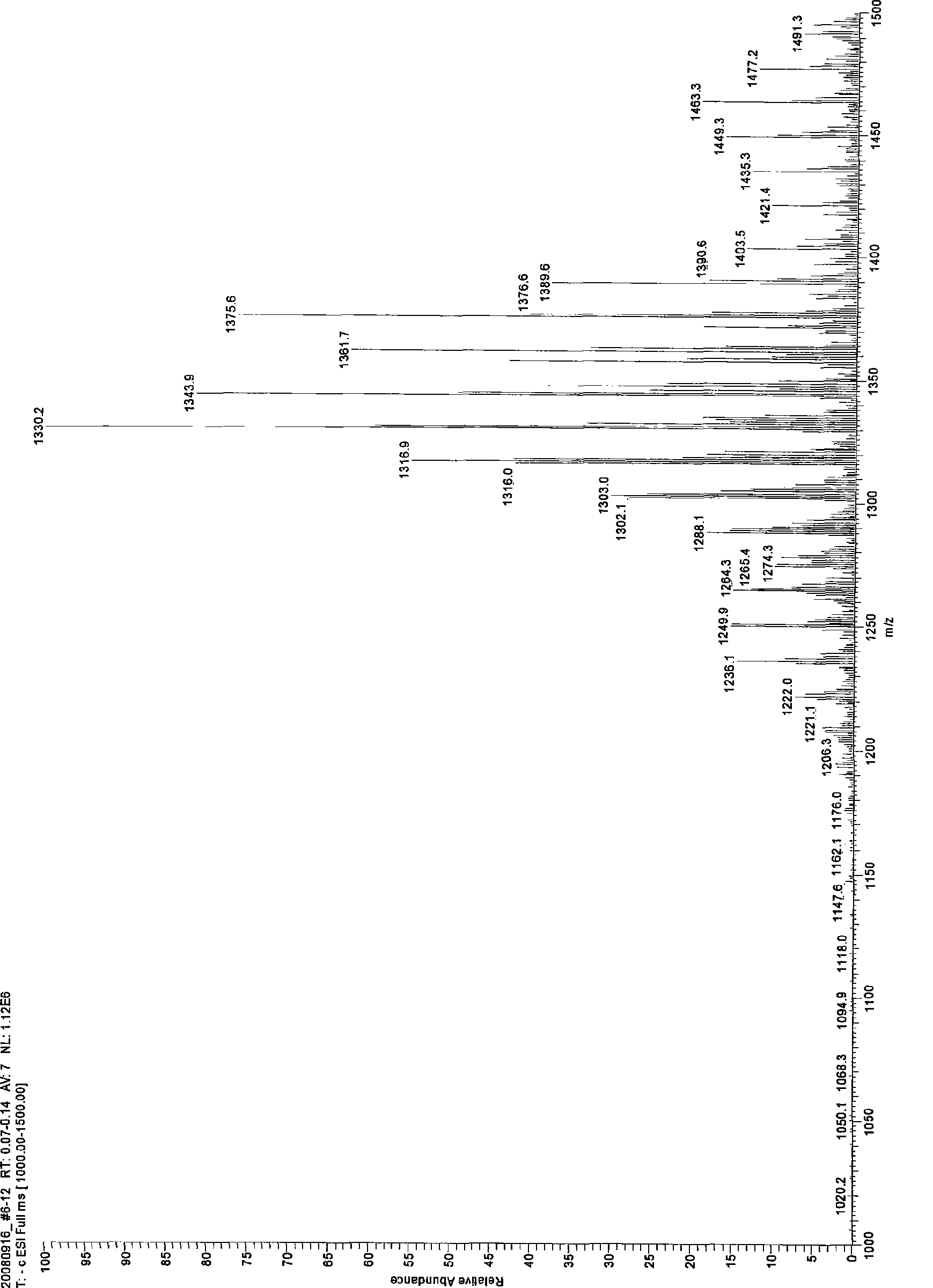
[0020] Embodiment 3: In the three-necked round-bottomed flask with reflux condenser and stirring, add 5g β-cyclodextrin (4.4mmol) and 57g dimethylformamide DMF and stir until clarification, then add potassium carbonate 1.0gK 2 CO 3 After stirring for 10 minutes, add 16.5 mL (185 mmol) of dimethyl carbonate, continue to stir, and react at 95° C. for 24 hours. The reaction process is monitored by TLC. After the reaction, K was removed by centrifugation 2 CO 3 and suspended solids, the pressure is 150-260Pa, the liquid phase temperature is 60-85°C, the solvent dimethylformamide DMF and unreacted dimethyl carbonate are distilled off under reduced pressure, the solution is concentrated to syrup, and added to it after cooling 100mL of acetone was stirred, and the acetone was filtered off, and the obtained powder solid was soaked in anhydrous ether for 24 hours, soaked twice to obtain 3.32 grams of the product, the yield of β-cyclodextrin was 66.4%, and the average degree of substi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com