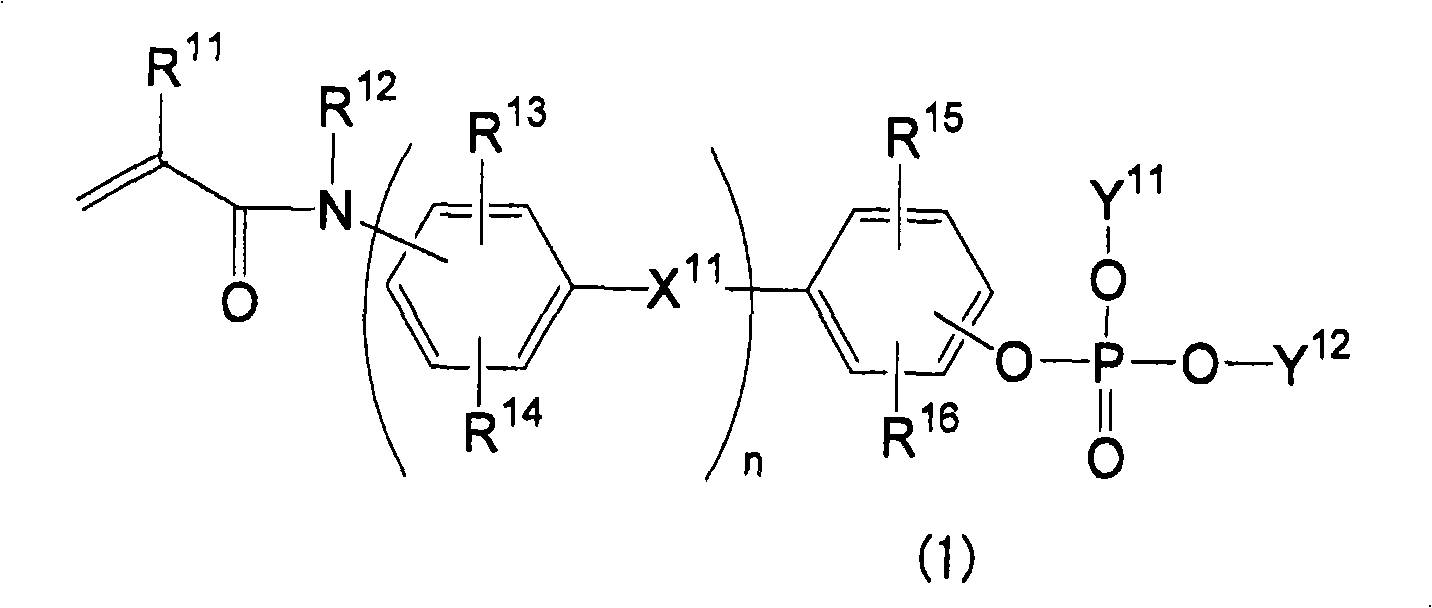
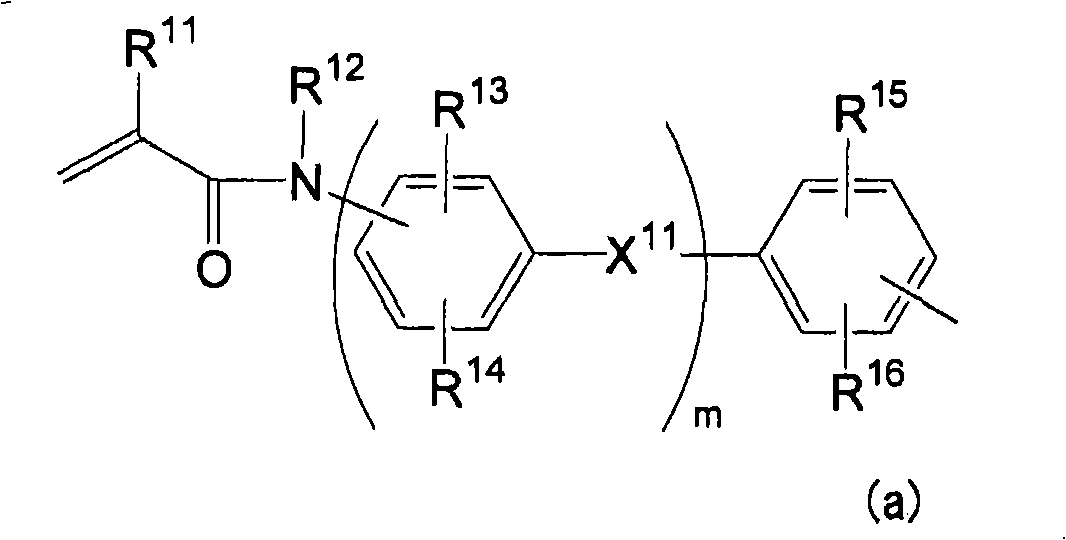
Phosphate ester compound, metal salt thereof, dental material, and demtal composition
A phosphate ester and compound technology, which is applied in the field of dental materials and dental compositions, phosphate ester compounds and their metal salts, can solve the problems of insufficient storage stability, operability, polymerizability, and adhesiveness, and material preservation. There are problems such as stability, and the effect of excellent polymerization is achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0238] The method for preparing the dental composition of the present invention is not particularly limited, but it is preferably prepared by a hitherto known method. That is, in the case of a composite resin for dental restoration, for example, a predetermined amount of a polymerizable compound, a polymerization initiator, a filler, and various additives added as required are weighed, mixed and kneaded, and a paste is prepared. The method of forming a composition, etc.
[0239] When using a polymerizable compound, in order to remove insoluble matter or foreign matter, it is preferable to perform purification by operations such as filtration before polymerization. In addition, in order to prevent generation of air bubbles in the cured product, it is preferable to sufficiently degas and defoam the composition under reduced pressure.
[0240] As a method of using the dental composition of the present invention, for example, in the case of a composite resin for dental restoratio...
Synthetic example 1
[0246]
[0247] Mix and dissolve 168.2 g (0.74 mol) of 2-(4'-aminophenyl)-2-(4'-hydroxyphenyl) propane and 350 g of N,N-dimethylacetamide, and keep at 40°C for 2 hours To the obtained solution, 77.0 g (0.74 mol) of methacryloyl chloride was dropped. After further heating at 50° C. for 1 hour, it was confirmed by liquid chromatography that the reaction was substantially completed, and then cooled to room temperature. After diluting by adding 300 ml of ethyl acetate, the aqueous phase was washed with water until it became neutral, and liquid separation was carried out, and then, the organic phase was taken out. The solvent was distilled off under reduced pressure, the mixture was concentrated, and the precipitated solid was collected by filtration. Carry out slag refining with methanol / water mixed solvent to obtain 2-(4'-methacryloylaminophenyl)-2-(4'-hydroxyphenyl) propane [following formula (2-ii-1 ) compound] 180.7 g (0.62 mol) of colorless powdery crystals.
[0248] Yie...
Synthetic example 2
[0260]
[0261] 32.7 g (0.30 mol) of p-aminophenol and 80 g of N,N-dimethylacetamide were mixed and dissolved, and 29.8 g (0.285 mol) of methacryloyl chloride was dropped into the resulting solution at 50° C. over 2 hours. After further heating at 50° C. for 2 hours, it was confirmed by liquid chromatography that the reaction was substantially completed, and then cooled to room temperature. Add 750g of ethyl acetate and 1300g of distilled water, wash with water, separate liquid, and further use NaHCO 3The organic phase was washed with aqueous solution. Then, 200 g of toluene was added to the organic phase, and the solvent was distilled off under reduced pressure. During the distillation, concentration was carried out while adding 400 g of toluene in two portions, and the distillation was terminated when a solid precipitated. After adding 500 g of toluene to the obtained solid to form slag, it was filtered and washed to obtain a wet body. Drying was carried out at 40° C. i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com