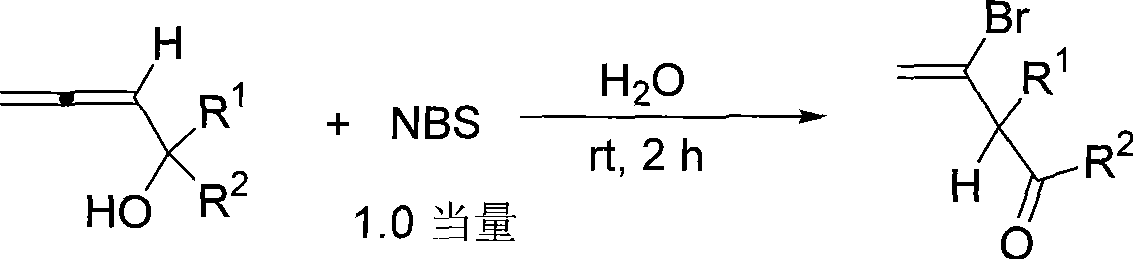
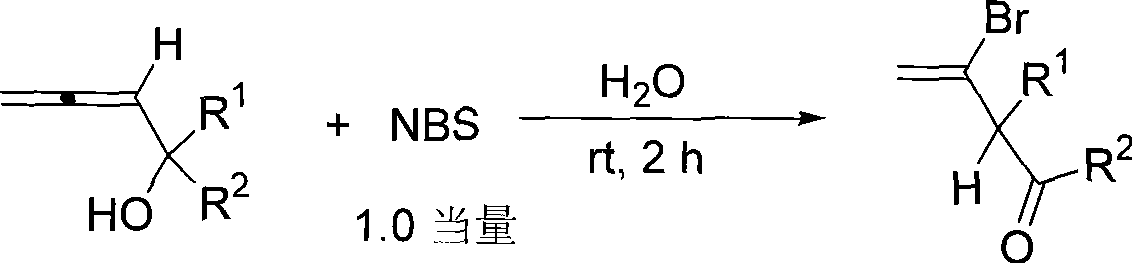Method for synthesizing Beta-bromo-Beta, Gamma-unsaturated olefin ketone
An unsaturated, enone-based technology, applied in the field of synthesis of β-bromo-β, to achieve a highly regioselective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 1-propadienylcyclohexanol (41.3mg, 0.30mmol) and water (4.0mL) successively at room temperature, then add N-bromosuccinimide (54.8mg, 0.31mmol), and react for 2 hours Add 1mL of saturated sodium thiosulfate solution to quench, extract with ether (3×10mL), wash once with 10mL of saturated saline, dry over anhydrous sodium sulfate, filter and concentrate, perform flash column chromatography (the column is rinsed with 10 drops of acetic acid in advance) washing), to obtain 37.8mg of product, the yield was 58%. The product is a colorless liquid.
[0018] 1 HNMR (300MHz, CDCl 3 )δ 5.70(d, J=1.8Hz, 1H), 5.58(d, J=1.8Hz, 1H), 3.45(dd, J 1 =10.8Hz,J 2 =3.3Hz, 1H), 2.71-2.50(m, 2H), 2.09-1.73(m, 5H), 1.62-1.28(m, 3H); 13 C NMR (75MHz, CDCl 3 )δ 210.9, 131.6, 118.7, 62.2, 43.4, 30.8, 29.6, 28.3, 24.6; IR (neat) v (cm -1 ) 2931, 2856, 1709, 1625, 1454, 1322, 1231, 1146; MS (70eV, EI) m / z (%): 218 (M + ( 81 Br), 26.3), 216 (M + ( 79 Br), 26.6), 67(100); HRMS calcd fo...
Embodiment 2
[0020] Add acetonitrile (4.0mL), 1-propadienyl cyclopentanol (37.8mg, 0.30mmol) and water (0.27mL) successively at room temperature, and then add N-bromosuccinimide (54.2mg, 0.30mmol ), add 1mL saturated sodium thiosulfate solution to quench after reacting for 2 hours, extract with ether (3×10mL), wash once with saturated brine 10mL, dry over anhydrous sodium sulfate, filter and concentrate, flash column chromatography (chromatographic column Rinse with 10 drops of acetic acid in advance) to obtain 33.1 mg of the product with a yield of 53%. The product is a colorless liquid.
[0021] 1 H NMR (300MHz, CDCl 3 )δ 5.69-5.64 (m, 2H), 3.34 (dd, J 1 =11.7Hz,J 2 =5.4Hz, 1H), 2.54-2.43(m, 1H), 2.40-2.27(m, 1H), 2.25-2.13(m, 1H), 2.11-1.88(m, 3H), 1.82-1.62(m, 2H ); 13 C NMR (75MHz, CDCl 3 )δ 207.3, 130.5, 119.4, 60.5, 41.9, 33.2, 27.2, 24.3; IR (neat) v (cm -1 ) 2940, 2865, 1716, 1630, 1449, 1425, 1297, 1204, 1129, 1069; MS (70eV, EI) m / z (%): 204 (M + ( 81 Br), 30.4), 202 (...
Embodiment 3
[0023] According to the method described in Example 1, the difference is that the substrates and reagents used are: 1-propadienyl cycloheptanol (45.4 mg, 0.30 mmol) and N-bromosuccinimide (53.5 mg, 0.30 mmol), reacted for 2 hours to obtain 42.7 mg of product, and the yield was 62%. The product is a colorless liquid.
[0024] 1 HNMR (300MHz, CDCl 3 )δ 5.77(dd, J 1 =2.4Hz,J 2 =1.2Hz, 1H), 5.61(d, J=2.4Hz, 1H), 3.67(dd, J 1 =10.2Hz,J 2 =4.2Hz, 1H), 2.57(ddd, J 1 =14.1Hz,J 2 =12.2Hz,J 3 =3.9Hz, 1H), 2.45(ddd, J 1 =14.1Hz,J 2 =6.0Hz,J 3 =3.9Hz, 1H), 2.07-1.80(m, 4H), 1.80-1.57(m, 2H), 1.53-1.21(m, 4H); 13 C NMR (75MHz, CDCl 3 )δ 214.2, 130.6, 118.1, 59.7, 42.7, 32.1, 27.4, 25.1, 24.6, 24.4; IR (neat) v (cm -1 ) 2930, 2857, 1712, 1633, 1619, 1465, 1446, 1411, 1357, 1326, 1309, 1234, 1190, 1160, 1126, 1103, 1078, 1061; MS (70eV, EI) m / z (%): 232(M + ( 81 Br), 8.96), 230 (M + ( 79 Br), 9.52), 151 (M + -Br, 79.2), 81(100); HRMS calcd for C 10 h 15 o 79 Br(M + ):...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com