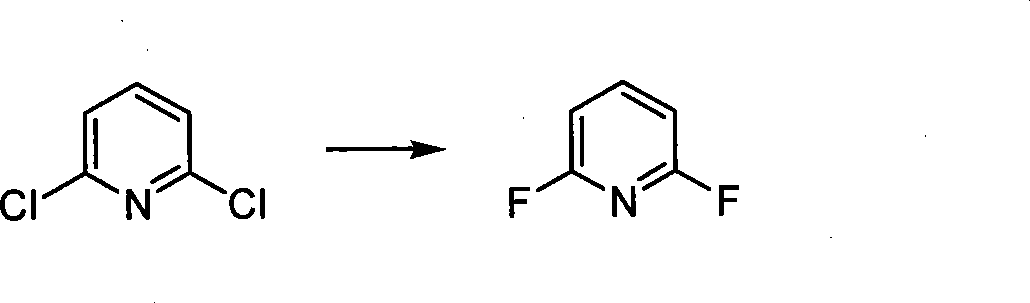
Method for synthesizing 2,6-difluoropyridine
A synthesis method and a technology for difluoropyridine are applied in 2 fields, can solve the problems of difficulty in reaching, high reaction temperature and high cost, and achieve the effects of safe and reliable production, high reaction yield and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
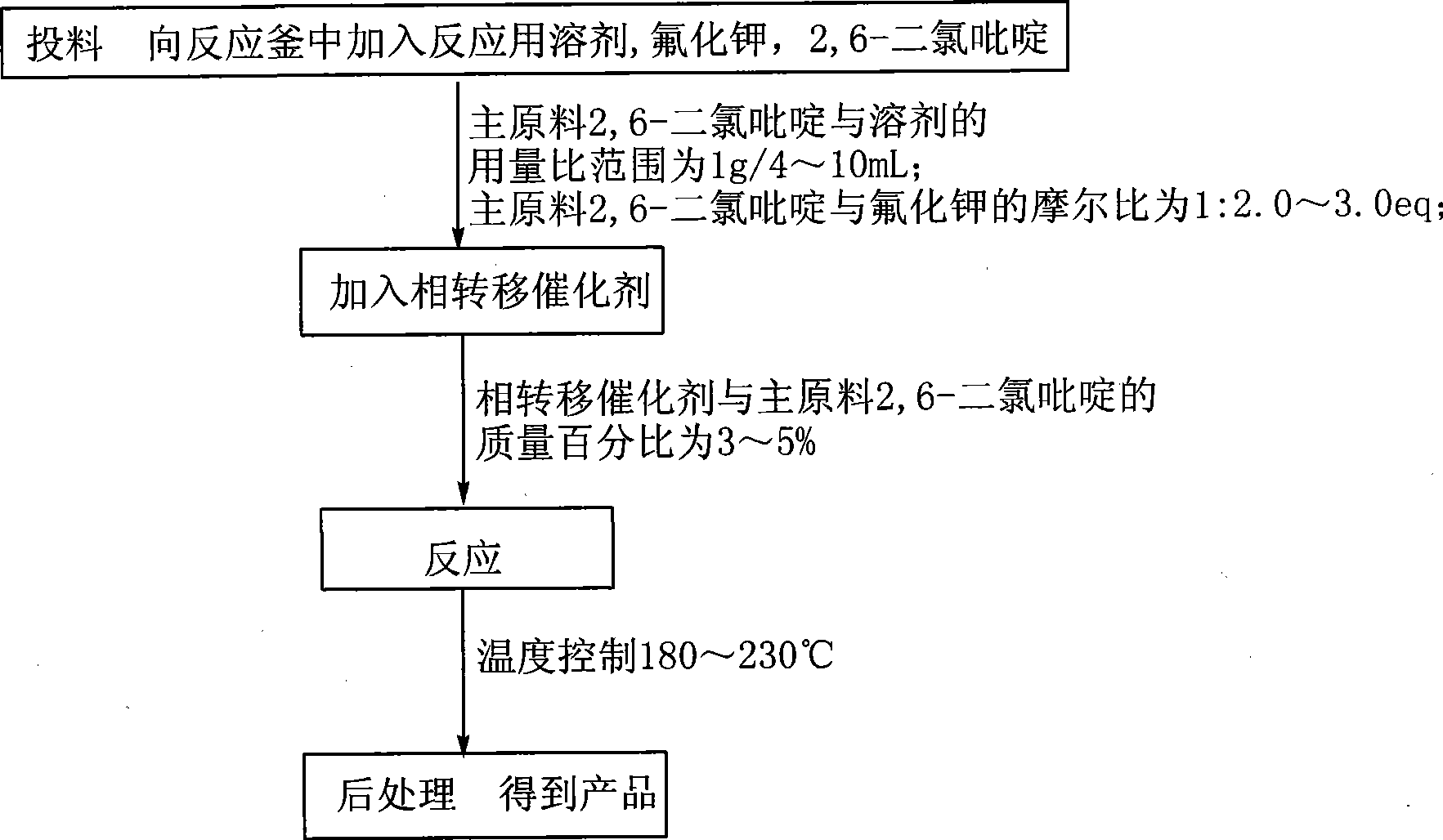
[0027] Into a 500L enamel kettle, pump 252.2kg of sulfolane (1g / 4ml) and 50kg (1.0eq) of 2,6-dichloropyridine into the system at one time, and then add 43.2g of potassium fluoride (2.2eq) and 1.5 at one time. kg18-crown-6(3%), stir for 20min, then heat the system temperature to 180~188℃, and react at 180~188℃ for 6h. The product will be transferred out of the reaction system by distillation during the reaction process. The driving reaction is completed, the fractions are washed with water, separated, the organic phase is dried, and after suction filtration, the filtrate is rectified, and the fractions at 122~124℃ are collected. The product is 30.4kg, the yield is 78.2%, and the gas chromatographic purity (GC): 99.5 %.
Embodiment 2
[0029] Into a 200L enamel kettle, pump 176.5kg of sulfolane (1g / 7ml) and 20kg (1.0eq) of 2,6-dichloropyridine into the system, and then add 15.7kg of potassium fluoride (2.0eq) and 1.0kg of four Ethylammonium bromide (5%), stir for 20min, then heat the system temperature to 190~200℃, and keep the temperature at 190~200℃ for 8h. The product will be transferred out of the reaction system by distillation during the reaction process. After driving the reaction to completion, the fractions are washed with water, separated, the organic phase is dried, and after suction filtration, the filtrate is rectified, and the fractions at 122~124℃ are collected. The product is 11.6kg, the yield is 74.6%, and the gas chromatography purity (GC): 99.1% .
Embodiment 3
[0031] Into a 200L enamel kettle, pump 154kg of dimethylsulfoxide (1g / 7ml) and 20kg (1.0eq) of 2,6-dichloropyridine into the system at one time, and then add 19.6kg of potassium fluoride (2.5eq) at one time. ) And 0.6kg tetraethylammonium bromide (3%), stir for 20min, then heat the temperature of the system to 210~220℃, and keep at 210~220℃ for 8h. The product is transferred out by distillation during the reaction. The reaction system drives the reaction to completion. The fractions are washed with water, separated into liquids, the organic phase is dried, and after suction filtration, the filtrate is rectified, and the fractions at 122~124℃ are collected. The product is 10.87kg, the yield is 69.9%, and the gas chromatography purity ( GC): 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com