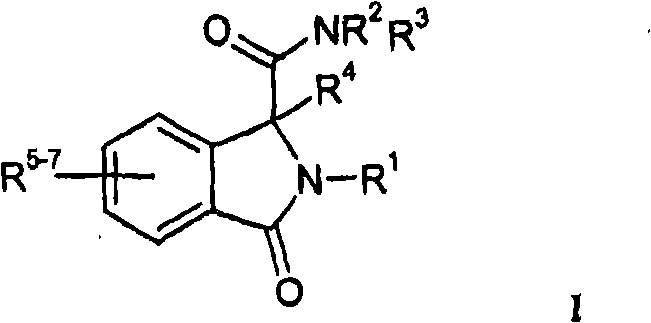
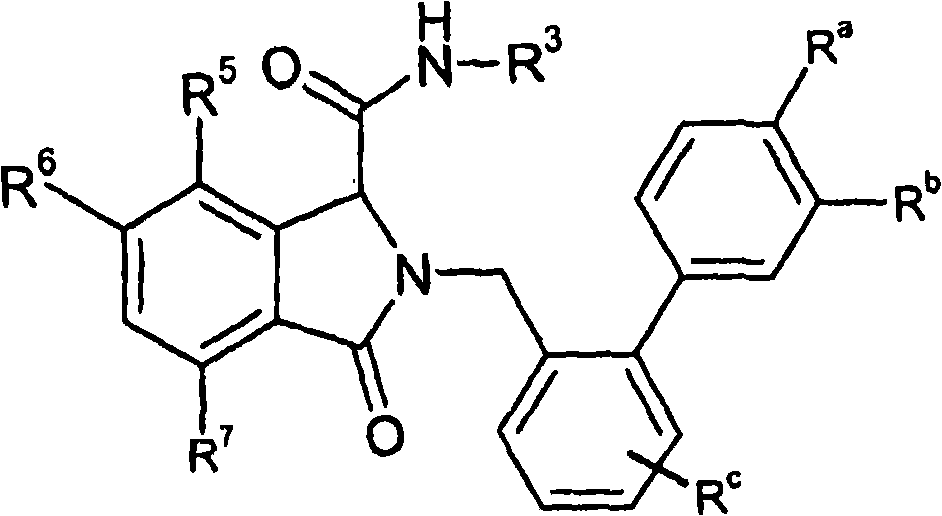
Isoindoline derivatives for the treatment of arrhythmias
A compound and alkyl technology, applied in the field of novel medicinal 3-oxoisoindoline-1-carboxamide compounds, can solve the problems of not giving references and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0975] 2-[2-(4-Chlorophenyl)propyl]-N-[(5-methylisoxazol-3-yl)methyl]-3-oxoisoindoline-1-carboxamide
[0976] A solution of 2-formylbenzoic acid (1.23g, 8.2mmol) in methanol (15ml) was treated with 2-(4-chloro-phenyl)-propylamine hydrochloride (16.9g, 8.2mmol) and triethylamine (1.14ml). The mixture was stirred at room temperature for 30 minutes. A solution of 3-isocyanomethyl-5-methyl-isoxazole from Preparation L was added and the mixture was stirred at room temperature for 16 hours. The mixture was concentrated, dissolved in 50 ml of dichloromethane and washed with 100 ml of saturated NaHCO 3 Solution wash. The organic phase was separated, dried over magnesium sulfate and evaporated. The remaining oil was purified using preparative HPLC to afford the title compound (0.903 g, 26% yield).
[0977] [M+1](ES)424.10
[0978] 1 H NMR (500MHz, CDCl 3 )δ7.46-7.61(m, 3H); 7.35-7.44(m, 1H); 7.07-7.27(m, 5H); 5.81(s, 1H); 4.78(s, 1H); 1H); 4.31-4.39 (m, 1H); 4.12-4.27 (m, 1H); ...
Embodiment 2
[0980] 2-(biphenyl-2-ylmethyl)-N-(tert-butyl)-5-hydroxy-4-methyl-3-oxoisoindoline-1-carboxamide
[0981] (i) N-(biphenyl-2-ylmethyl)-N-[2-(tert-butylamino)-1-(2-furyl)-2-oxoethyl]but-2-yne amide
[0982] (Biphenyl-2-ylmethyl)amine (23.78mmol, 4.36g) was dissolved in methanol, 2-furfural (23.78mmol, 2.29g) and but-2-ynoic acid (23.78mmol, 2.00g) were added. The mixture was stirred at room temperature for 30 minutes. Isocyano-tert-butane (23.7g, 1.98g) was added and the mixture was stirred at room temperature overnight. The solvent was removed by evaporation. The product was used in the next step without further purification.
[0983] (ii) 2-(biphenyl-2-ylmethyl)-N-(tert-butyl)-5-hydroxy-4-methyl-3-oxoisoindoline-1-carboxamide
[0984] N-(biphenyl-2-ylmethyl)-N-[2-(tert-butylamino)-1-(2-furyl)-2-oxoethyl]butyl from step (i) above -2-Ynamide (23.0mmol, 9.87g) was dissolved in xylene (200ml), and ytterbium(III) trifluoromethanesulfonate (2.30mmol, 1.43g) was added. The mixtu...
Embodiment 3
[0988] (R or S) 2-(biphenyl-2-ylmethyl)-N-(tert-butyl)-5-hydroxy-4-methyl-3-oxoisoindoline-1-carboxamide
[0989] (S or R) 2-(biphenyl-2-ylmethyl)-N-(tert-butyl)-5-hydroxy-4-methyl-3-oxoisoindoline-1-carboxamide
[0990] Separation of 2-(biphenyl-2-ylmethyl)-N-(tert-butyl)-5-hydroxyl by preparative HPLC using Reprosil 20×250 mm chiral column with 40% isopropanol in heptane as mobile phase - Enantiomer of 4-methyl-3-oxoisoindoline-1-carboxamide (Example 2) (0.20 g, 0.47 mmol) to give the (+) enantiomer of the title compound (0.10 g) (E1 ) and (-) enantiomer (0.10 g) (E2).
[0991] (+) Enantiomers:
[0992] HRMS: Yes (C 27 h 28 N 2 o 3 +H) + Calculated: 429.2178; found (ES[M+H]+): 429.2166.
[0993] (-) enantiomer:
[0994] HRMS: Yes (C 27 h 28 N 2 o 3 +H) + Calculated: 429.2178; found (ES [M+H]+): 429.2147.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com