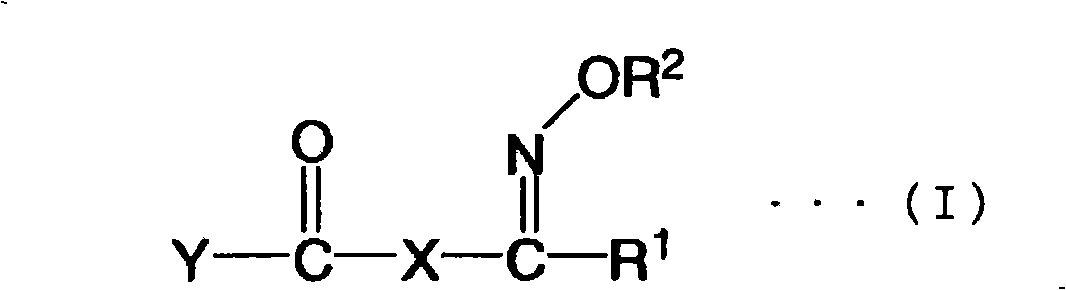
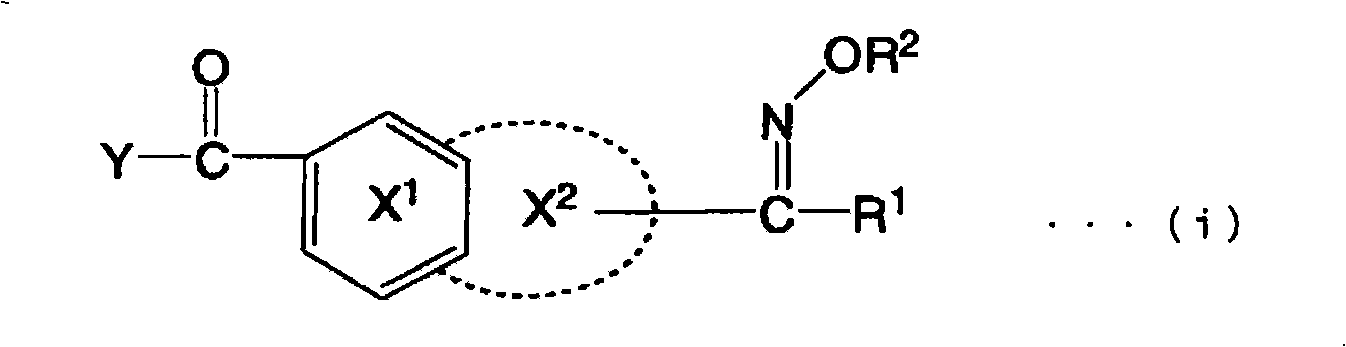
Oxime ester compound, photopolymerization initiator, photopolymerizable composition, color filter, and liquid crystal display device
A photopolymerization initiator and photopolymerizable technology, applied in the field of photopolymerization initiators and oxime ester compounds, can solve the problem that the accuracy, reliability, productivity of color filters cannot be obtained, and the resolution adhesion and developability are changed. problems such as poor durability, to achieve the effects of excellent durability, low cost, and a wide range of application technologies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0310] The following examples and comparative examples are given to further describe the present invention in detail, but the present invention is not limited to the following examples unless the gist is exceeded.
Synthetic example 1
[0311] [Synthesis example 1 (synthesis of epoxy acrylate resin having carboxyl group)]
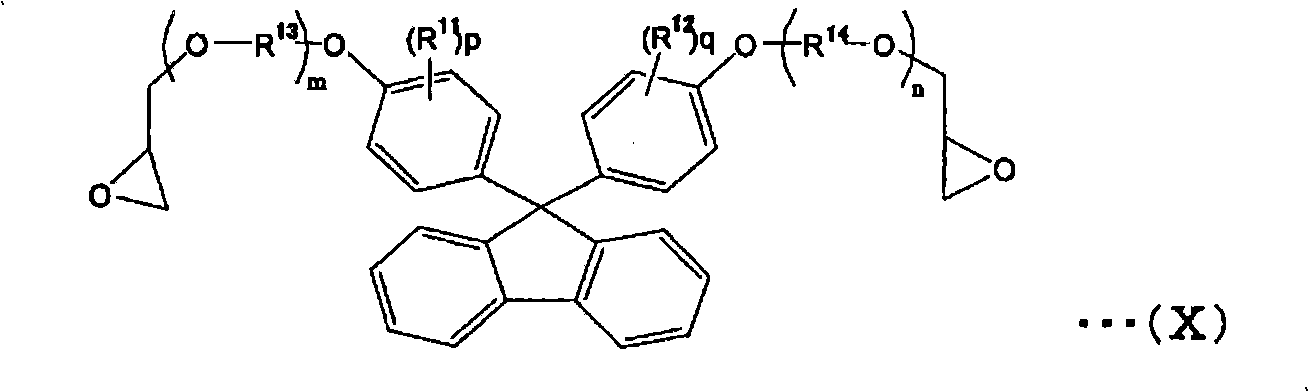
[0312] In a 500mL four-necked flask, add 245g of bisphenol fluorene type epoxy resin (epoxy equivalent weight 245) represented by the following formula (a-1), 110mg of tetramethylammonium chloride, 100mg of 2,6-di-tert-butyl-4 -Methyl phenol, 72.0g of acrylic acid and 300g of propylene glycol monomethyl ether acetate are blown into the air at a speed of 25ml / minute, while heating and dissolving at 90°C to 100°C.
[0313] Next, the temperature was gradually raised while the solution was cloudy, and it was heated to 120° C. to completely dissolve it. Here, the solution gradually became transparent and viscous, and stirring was continued in this state. During this period, measure the acid value, continue heating and stirring until the acid value is less than 1.0 mg-KOH / g. It takes 12 hours for the acid value to reach the target value. Then cooled to room temperature to obtain bisphenol flu...
Synthetic example 2
[0318] [Synthesis example 2 (synthesis of epoxy acrylate resin having carboxyl group)]
[0319] Use 261g of bisphenol fluorene type epoxy resin (epoxy equivalent weight 261) represented by the following formula (a-2) instead of the bisphenol fluorene type epoxy resin represented by formula (a-1), except that it is the same as Synthesis Example 1 The synthesis was carried out to obtain a resin with an acid value of 103 mg-KOH / g and a molecular weight of 4400.
[0320] [chemical 16]
[0321]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
| Acid value | aaaaa | aaaaa |
| Acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com