Positively charged water-soluble prodrugs of prostaglandins and related compounds with very high skin penetration rates
A technology of prostaglandin and prostaglandin, which is applied in the field of prodrugs of positively charged water-soluble prostaglandins and related compounds with fast skin penetration speed, can solve problems such as destruction, pain, high cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
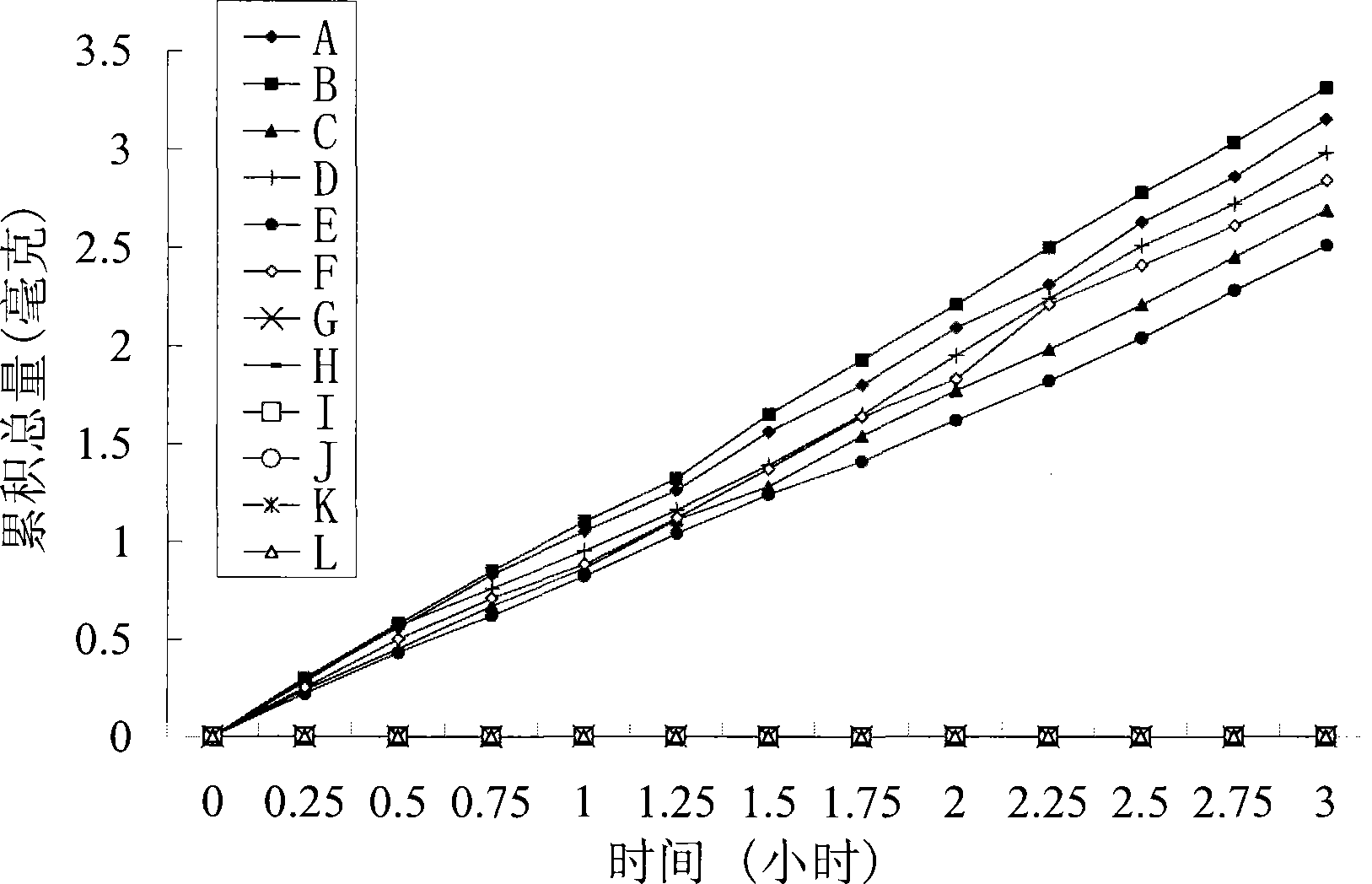
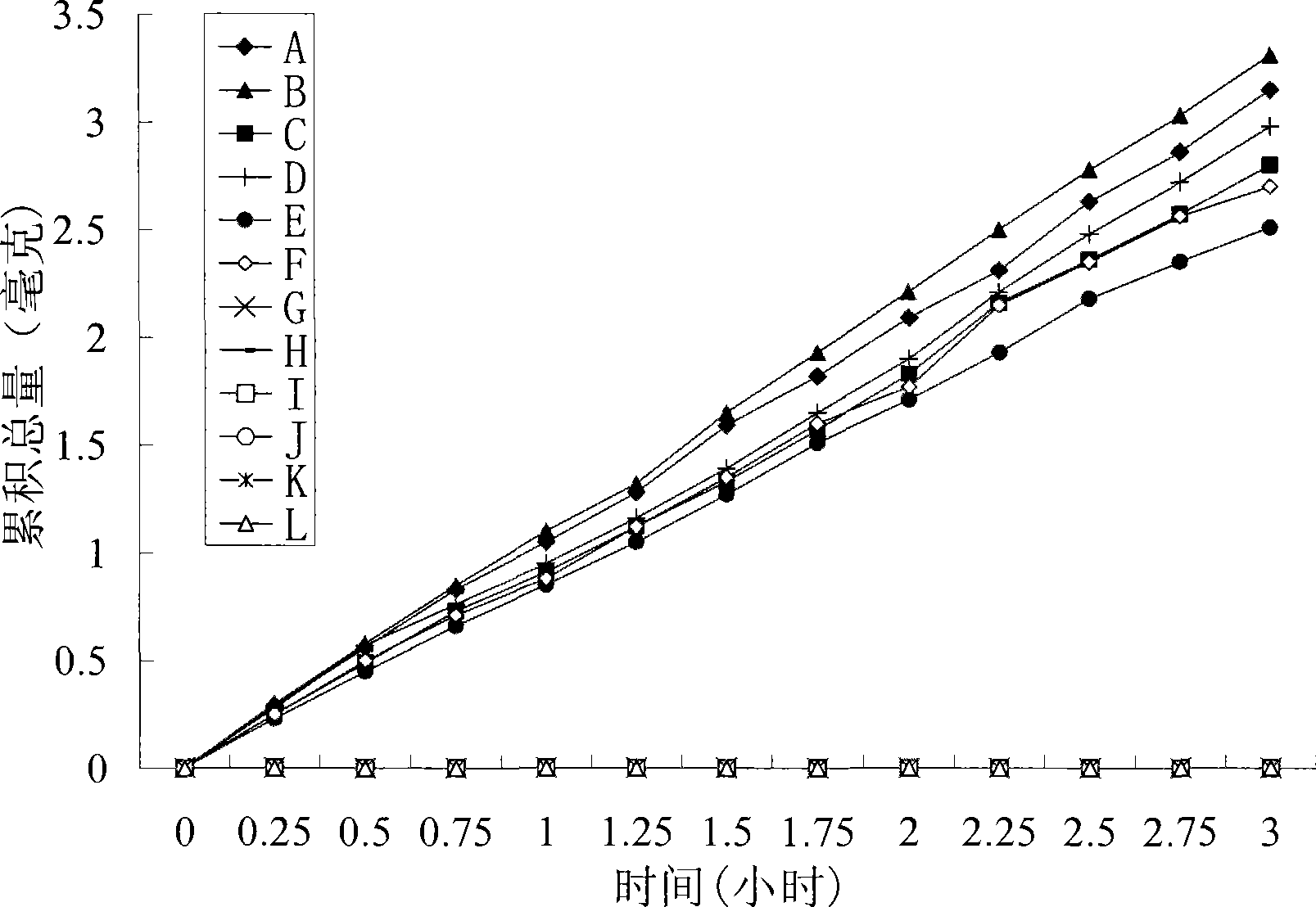
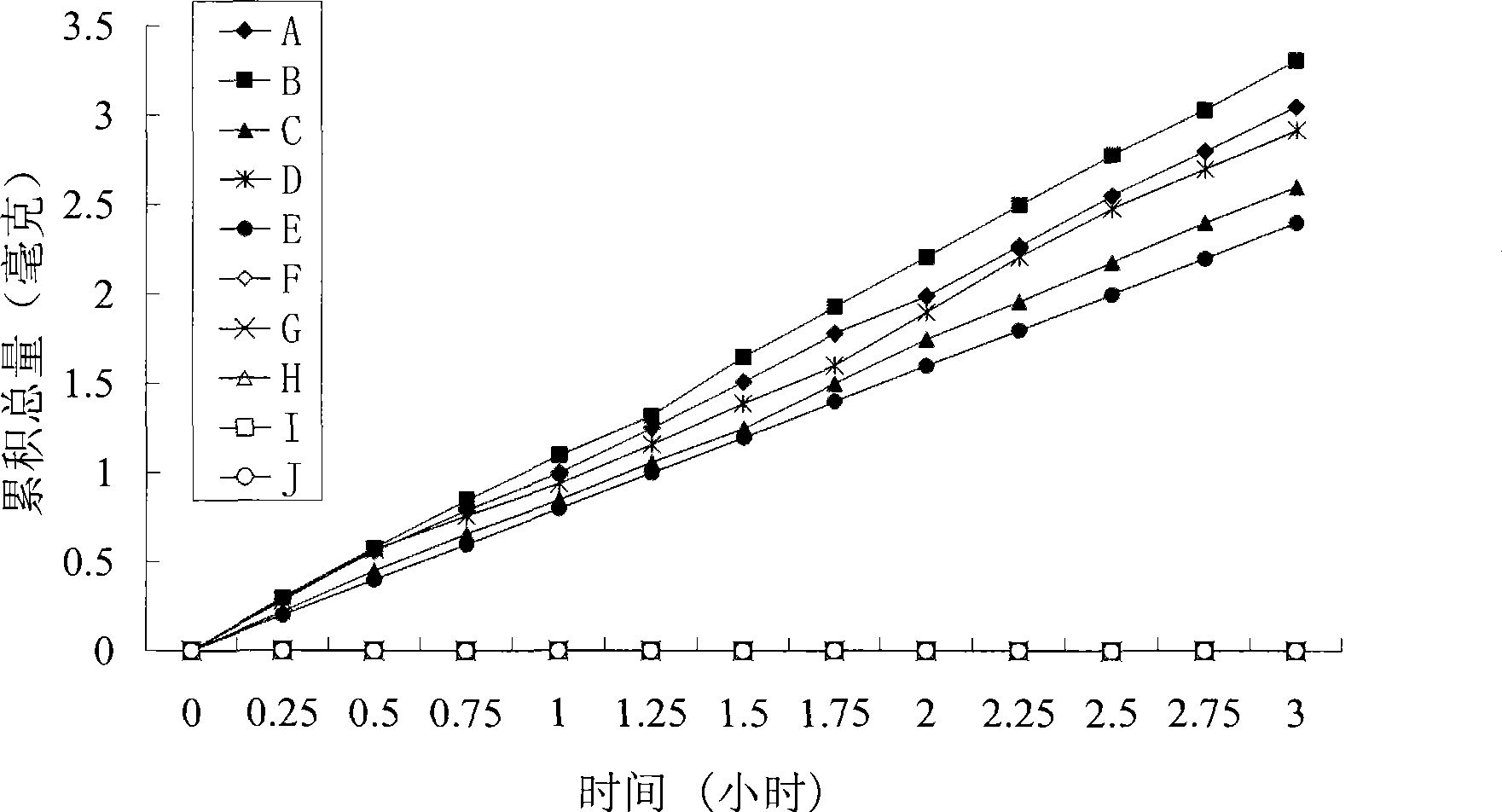
Image
Examples
Embodiment approach
[0064] N, the preparation method of N-diethylaminoethyl 11,15-diacetoxy-9-ketone-5,13-prostadienoic acid-1-amide acetate
[0065] 43.7 g (0.1 mol) of 11,15-diacetoxy-9-keto-5,13-prostadienoic acid were dissolved in 300 ml of chloroform. To the reaction mixture was added 20.6 g of N,N'-dicyclohexylcarboimide. To the reaction mixture was added 11.7 g of N,N-diethylaminoethylamine hydrogen bromide. The mixture was stirred at room temperature for 3 hours. The solids were removed by filtration. The chloroform solution was washed twice with 5% aqueous sodium bicarbonate, 100 ml each time, and washed three times with water, 100 ml each time. The organic layer was dried over anhydrous sodium sulfate. Sodium sulfate was removed by filtration. 6 g of acetic acid was added to the reaction mixture with stirring. 200ml of hexane was added. The solid product was collected by filtration. After drying, 45 g of the hygroscopic target product was obtained with a yield of 85.8%. Solubil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com