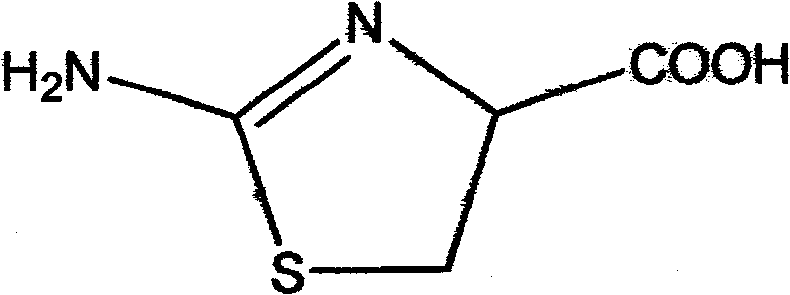
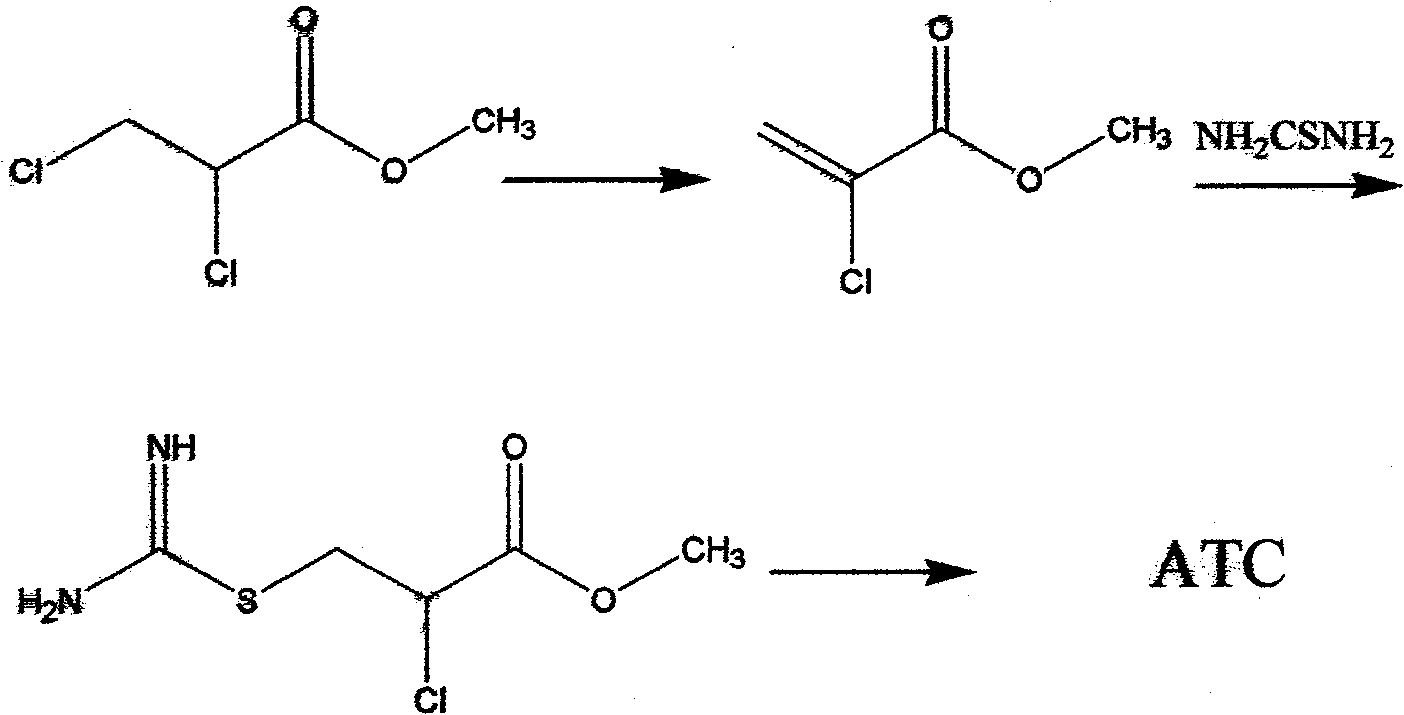
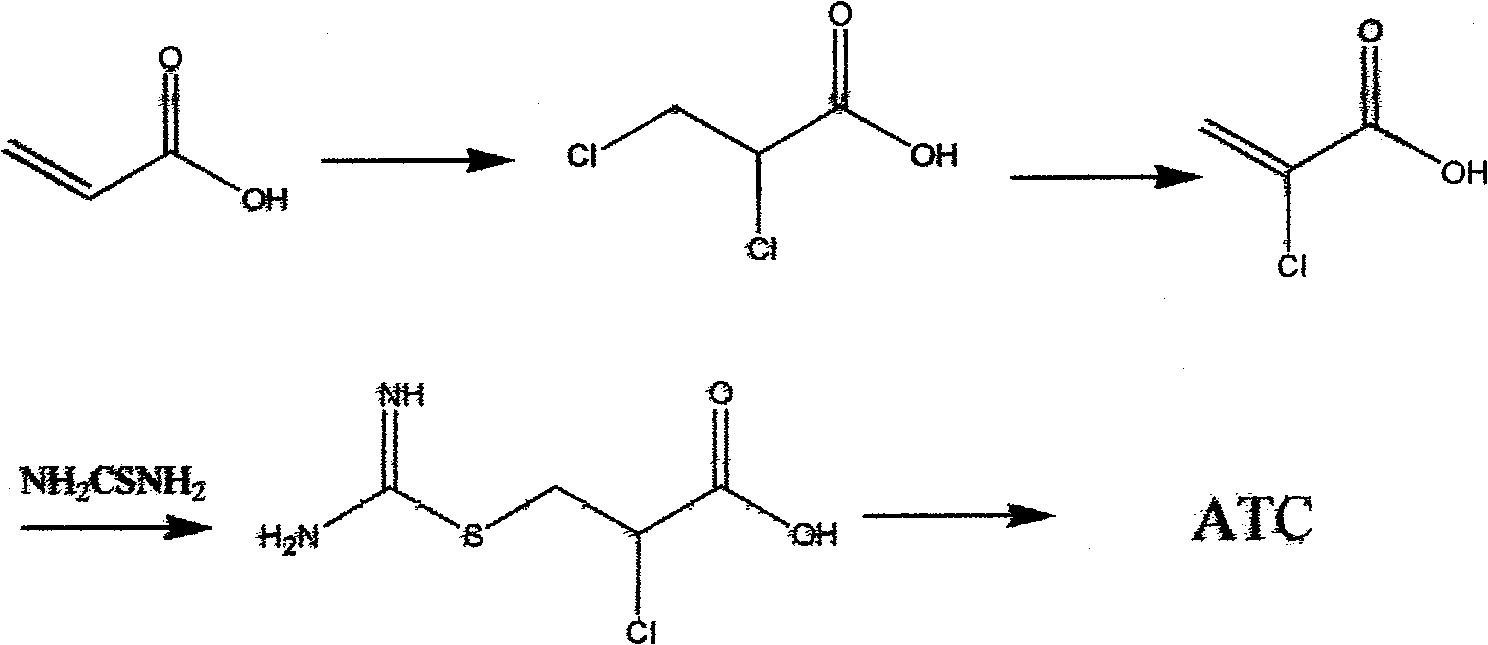
Novel method for synthesizing 2-amino-2-thiazoline-4-carboxylic acid
A synthesis method and thiazoline technology are applied in the synthesis field of fermentation precursor 2-amino-2-thiazoline-4-carboxylic acid, and can solve the problems of low product quality, low yield, complicated steps and the like, and achieve yield High, improve product quality, the effect of simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 80g (0.5mol) of 3-chloro-alanine hydrochloride and 300ml water into a 500ml three-necked flask and stir to dissolve, then add 41g (0.5mol) of sodium thiocyanate solid into the reaction flask, and continue stirring at room temperature Mix for 15 minutes, adjust the pH to 8.0-9.0 with sodium carbonate, heat in a water bath to 60°C, and continue to use sodium carbonate to maintain the pH, consume a total of 29-30g of sodium carbonate, and react at 60°C for 3 hours to obtain 3-chloro-2-thiourea A pale yellow reaction solution of propionic acid. Concentrated hydrochloric acid was added dropwise to the reaction solution with a constant pressure dropping funnel to adjust the pH to 6.0-7.5. Then raise the temperature to 90°C, keep the reaction at 90°C, and carry out the ring-closing reaction for 2 hours. After the reaction is completed, when the temperature is lowered to 40°C, in order to speed up the crystallization, a small amount of crystal seeds or friction wall can be ...
Embodiment 2
[0026] Add 52.6g (0.5mol) of 3-hydroxy-alanine hydrochloride and 300mol water into a 500ml three-necked flask and stir to dissolve, then add 41g (0.5mol) of sodium thiocyanate solid into the reaction flask, and stir and mix at room temperature for 15min , adjust the pH to 8.5-9.0 with about 29g of sodium carbonate, raise the temperature to 65°C, and keep the temperature to continue the reaction for 2.8h to obtain a light yellow reaction solution of 3-hydroxy-2-thioureidopropionic acid, add dropwise to the reaction solution Concentrated hydrochloric acid, adjust the pH to 7.0-7.5, then raise the temperature to 100°C, keep warm for 1.8h for closed-loop reaction. After the reaction is complete, cool down to 38°C, add seed crystals to induce crystallization, then continue to cool down to 5-10°C, keep for 32 minutes, then filter, wash the product with 100ml of cold water to obtain 2-amino-2-thiazoline-4-carboxylic acid, Vacuum-dried at 50-60°C to obtain 69.5 g of the finished produ...
Embodiment 3
[0028] Add 84g (0.5mol) of 3-bromo-alanine hydrochloride and 300ml water into a 500ml three-necked flask and stir to dissolve, add 41g (0.5mol) of sodium thiocyanate solid, stir and mix at room temperature for 10min, adjust the pH with sodium carbonate To 8.0-8.5, the temperature was raised to 55°C, and the pH was maintained, and the reaction was carried out at 55°C for 3.2h. A reaction solution of 3-bromo-2-thioureidopropionic acid was obtained. Concentrated hydrochloric acid was added dropwise to the reaction solution to adjust the pH to 6.0-6.5. The temperature was raised to 85°C, and the ring-closing reaction was maintained for 2.2 hours. After the reaction is completed, when the temperature is lowered to 45°C, crystallization is induced on the wall of the friction vessel, and the temperature is further lowered to 4-6°C, kept for 35 minutes, filtered, and the product is washed with 100ml of cold water to obtain 2-amino-2-thiazoline-4-carboxylic acid, Vacuum-dried at 50-6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com