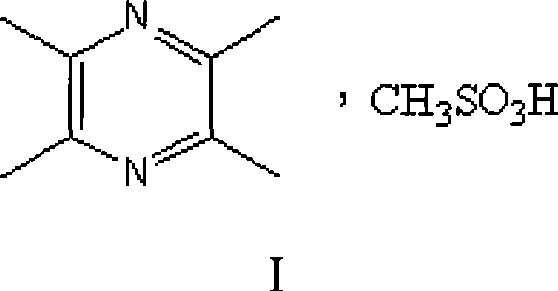
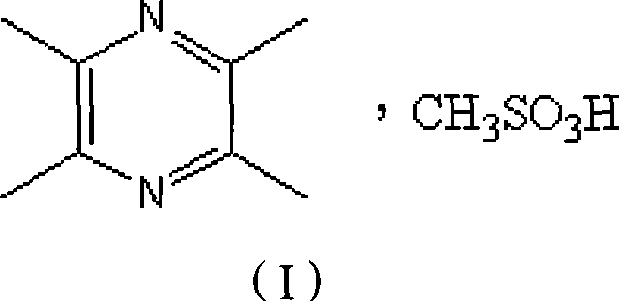
Ligustrazine salt
A technology of ligustrazine and methanesulfonic acid, applied in the salt field of ligustrazine, can solve problems such as unfavorable preservation and preparation preparation, easy sublimation of hygroscopicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1. Preparation of ligustrazine mesylate
[0018] Preparation of ligustrazine: Dissolve 100 g of ligustrazine hydrochloride (commercially available) in 1500 mL of water, adjust the solution to pH 12 with 20% NaOH aqueous solution, a large amount of white solid precipitates out, extract with 1500 mL of dichloromethane, separate the organic layer, anhydrous It was dried over sodium sulfate, and the solvent was evaporated to obtain 57.5 g of free ligustrazine.
[0019] Preparation of ligustrazine mesylate: Dissolve 10 g of ligustrazine mesylate in 300 mL of ethyl acetate, add 4.8 ml of methanesulfonic acid dropwise while stirring, and continue stirring for 30 minutes after the addition. A solid precipitates out. Heat to reflux until the solid is completely dissolved. Cooling, crystallization, standing to obtain white needle-like crystals, filtering, washing with ethyl acetate, and vacuum drying at 60°C for 3 hours to obtain 13.4 g of white needle-like ligustrazine mesyl...
Embodiment 2
[0020] Example 2: Comparative experiment of sublimation of ligustrazine mesylate, ligustrazine phosphate and ligustrazine hydrochloride
[0021] Weigh 1g of ligustrazine phosphate (commercially available, Chinese Pharmacopoeia 2005 standard), ligustrazine hydrochloride (commercially available), and ligustrazine mesylate (prepared by the method in Example 1) respectively, and spread them on the same caliber dry to constant weight. In the weighing bottle, prepare 15 samples of each salt and dry them in an oven under normal pressure at 60℃. Weigh each salt at 0.5hr, 1.5hr, 2.5hr, 3.5hr, and 4.5hr time points. Measure the weight of three samples for each salt, record the weight loss of the sample, calculate the weight loss percentage, and use the average value to evaluate the weight loss of the salt at the time point as a percentage of the original weight. The experimental results are shown in the table 1 shown.
[0022] Weight loss percentage = (weight before the experiment-weight af...
Embodiment 3
[0026] Example 3. Preparation of Ligustrazine Mesylate Tablets
[0027] It can be prepared according to the general process of the tablet, such as the preparation of 45mg / tablet of ligustrazine mesylate tablets
[0028] prescription:
[0029]
[0030] Process: Take ligustrazine mesylate, microcrystalline cellulose, and sodium carboxymethyl starch, mix uniformly, add 2% hydroxypropyl methyl cellulose to prepare soft material, granulate with 16 mesh sieve, dry at 60°C for 4 hours, Turn it over from time to time, use a 16-mesh sieve to size the dry granules, add magnesium stearate and mix evenly, then press to obtain a tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com