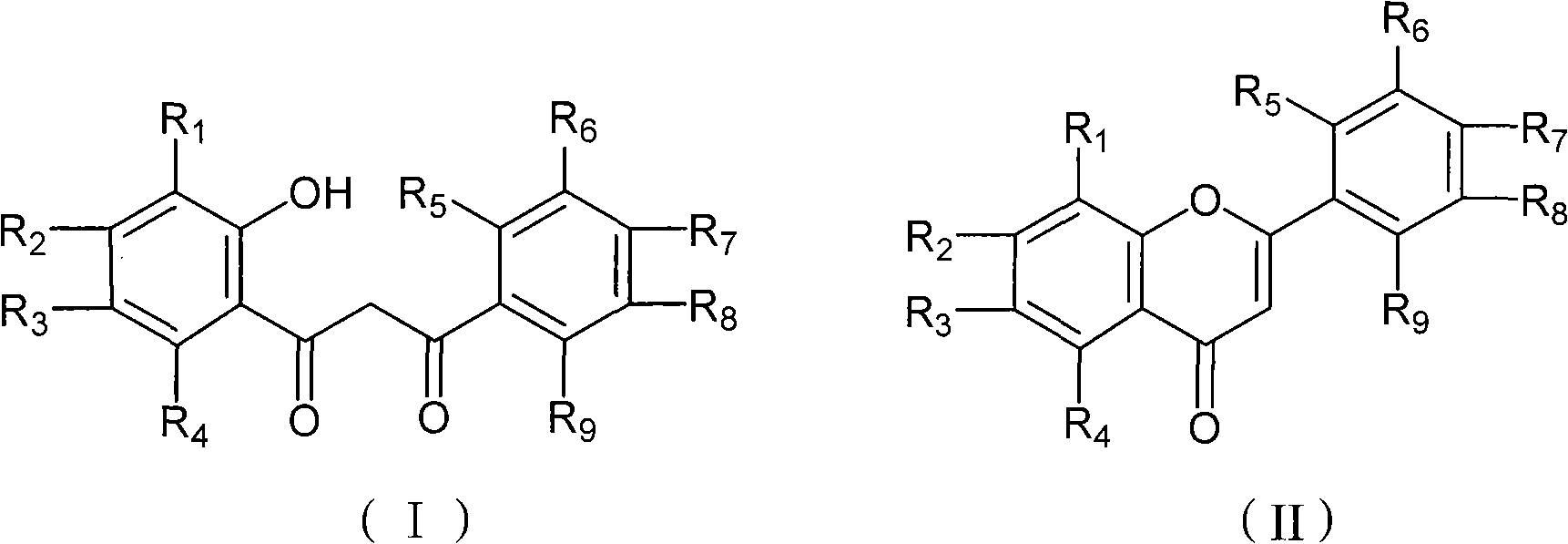
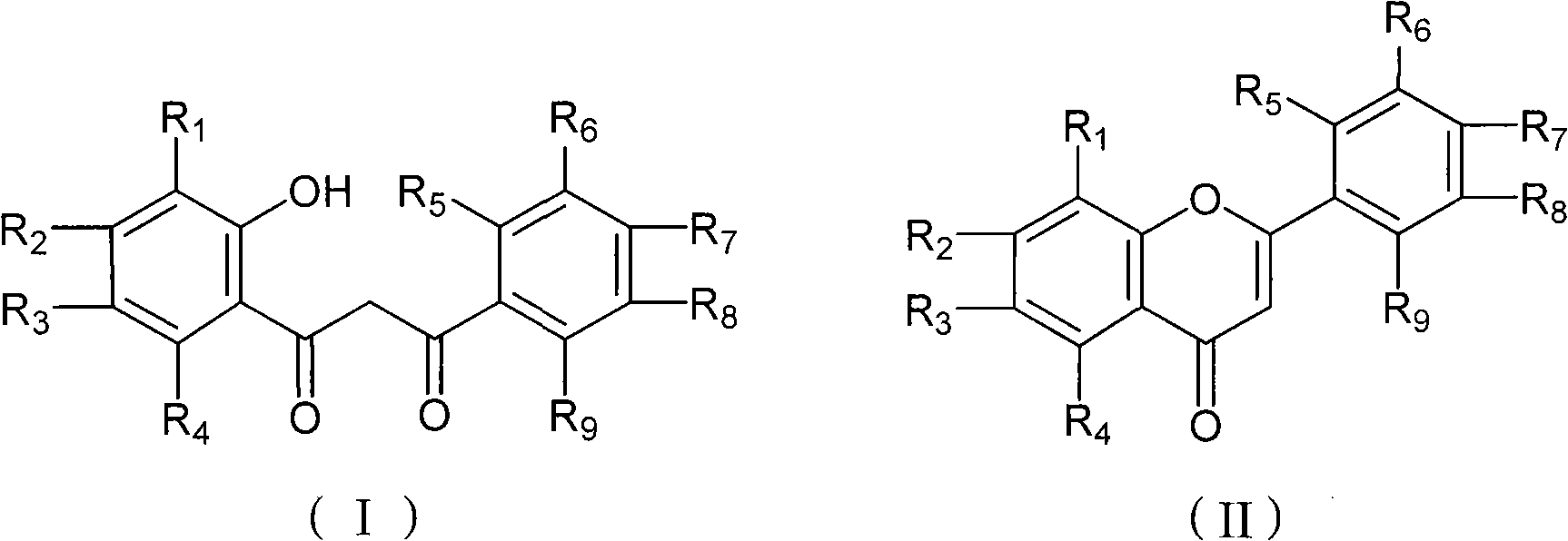
Chemical synthesis method of flavonoid compound
A technology of chemical synthesis of flavonoids, which is applied in the field of chemical synthesis of flavonoids, can solve the problems of being in the research and exploration stage, difficult to recycle, and environmental pollution, and achieve the effects of environmental friendliness, no three wastes, and reasonable process routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the preparation of flavone
[0024] Add 50mL of nitromethane 1-(2'-hydroxyphenyl)-3-phenyl-1,3-propanedione into a 100mL four-necked reaction flask equipped with mechanical stirring, drying tube, thermometer and dropping funnel (4.8g, 20mmol) and gallium trifluoromethanesulfonate (516mg, 1mmol), the temperature was raised to 80°C for 2 hours, and the progress of the reaction was tracked by TLC. After the reaction, the reaction mixture was poured into 100 mL of water, extracted three times with 20 mL of dichloromethane × 3, the organic layers were combined, dried over anhydrous sodium sulfate, the solvent was removed by rotary evaporation, and then recrystallized with ethanol to obtain 4.28 g of flavonoids, white Solid, melting point 96-97°C, yield 96.4%. The aqueous phase was concentrated under reduced pressure to obtain white crystals, which were heated to 200°C under vacuum to obtain white crystals (Ga(OTf) 3 ) 475 mg, 92%.
Embodiment 2
[0025] Embodiment 2: the preparation of flavone
[0026] Nitromethane 50 mL, 1-(2'-hydroxyphenyl)-3-phenyl-1,3-propanedione (4.8 g, 20 mmol), bismuth trifluoromethanesulfonate (656 mg, 1 mmol). Other operations are the same as in Example 1. 4.19 g of flavonoids were obtained with a yield of 94.4%. 590.4 mg of bismuth trifluoromethanesulfonate was recovered, and the yield was 90%.
Embodiment 3
[0027] Embodiment 3: the preparation of flavone
[0028] Nitromethane 50 mL, 1-(2'-hydroxyphenyl)-3-phenyl-1,3-propanedione (4.8 g, 20 mmol), copper trifluoromethanesulfonate (362 mg, 1 mmol). Other operations are the same as in Example 1. 4.01 g of flavonoids were obtained with a yield of 90.3%. 307.5 mg of copper trifluoromethanesulfonate was recovered, and the yield was 84.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com