Epitope minimum motif peptide of human zona pellucida protein
A technology of zona pellucida protein and motifs, applied in the fields of bioengineering and immunology, can solve problems such as false positive results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
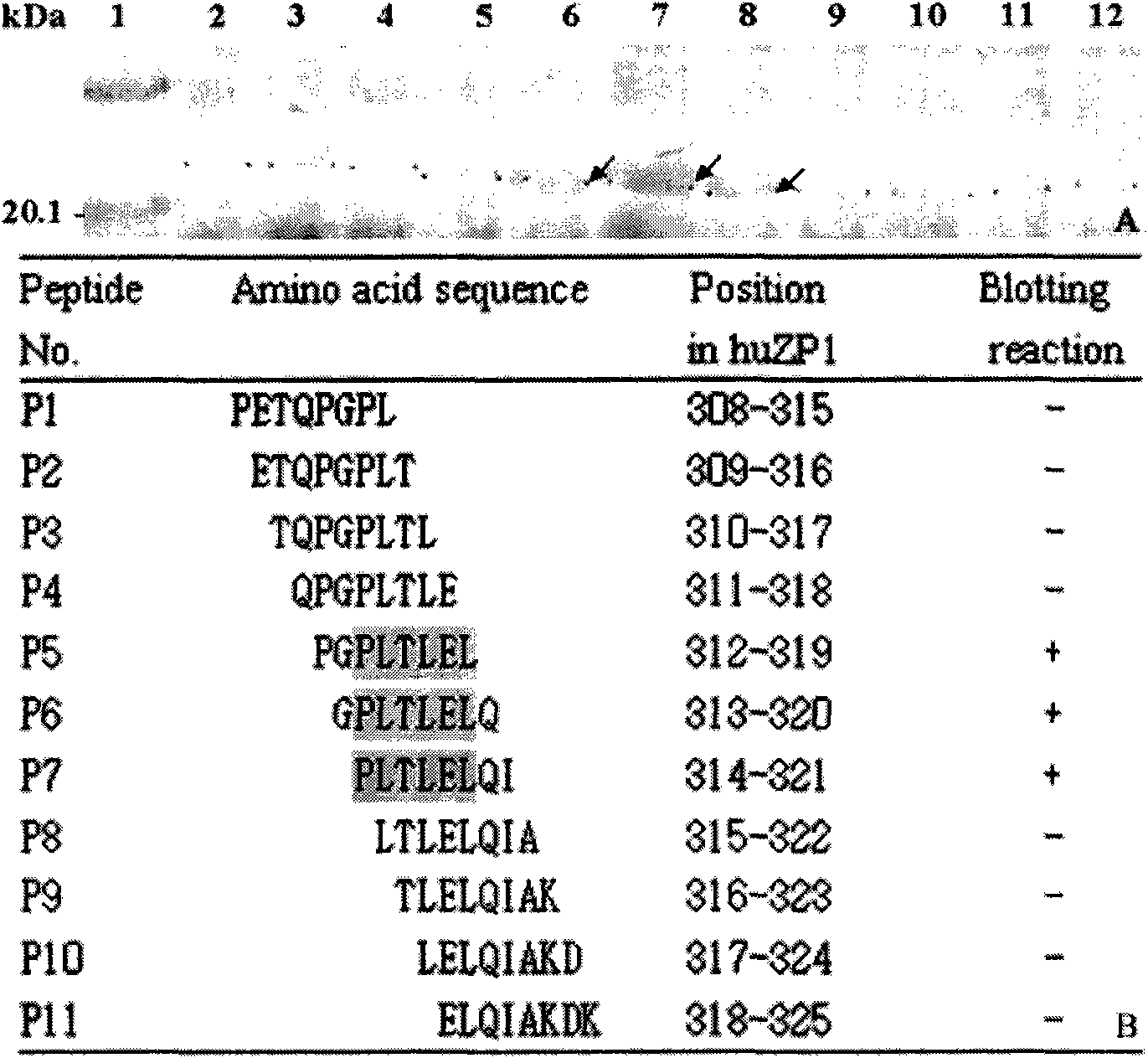
[0019] Example 1: Human egg zona pellucida protein-1 (huZP1) epitope 313-321 Minimal motif identification
[0020] Materials and methods:
[0021] 1. The heat-inducible expression plasmid pXXGST-1 was constructed by the inventor of this patent (patent application number: 200710173305.2). Escherichia coli BL21 (DE3) strain was preserved by the State Key Laboratory of Genetic Engineering, Fudan University.
[0022] 2. Rabbit anti-pig ZP IgGs antibody and rabbit anti-huZP3a and huZP3b antiserum were prepared and preserved by Shanghai Institute of Family Planning Science.
[0023] 3. Restriction enzymes BamH I, Sal I and T4 DNA ligase were purchased from Japan TaKaRa Biotechnology Company, protein low molecular weight standards, horseradish peroxidase-labeled goat anti-rabbit secondary antibody (IgG / HRP), diaminobenzidine (DAB) and nitrocellulose membranes were purchased from Huamei Bioengineering Company.
[0024] 4. QIAprep spin miniprep Kit plasmid extraction kit, QIAquick ...
Embodiment 2
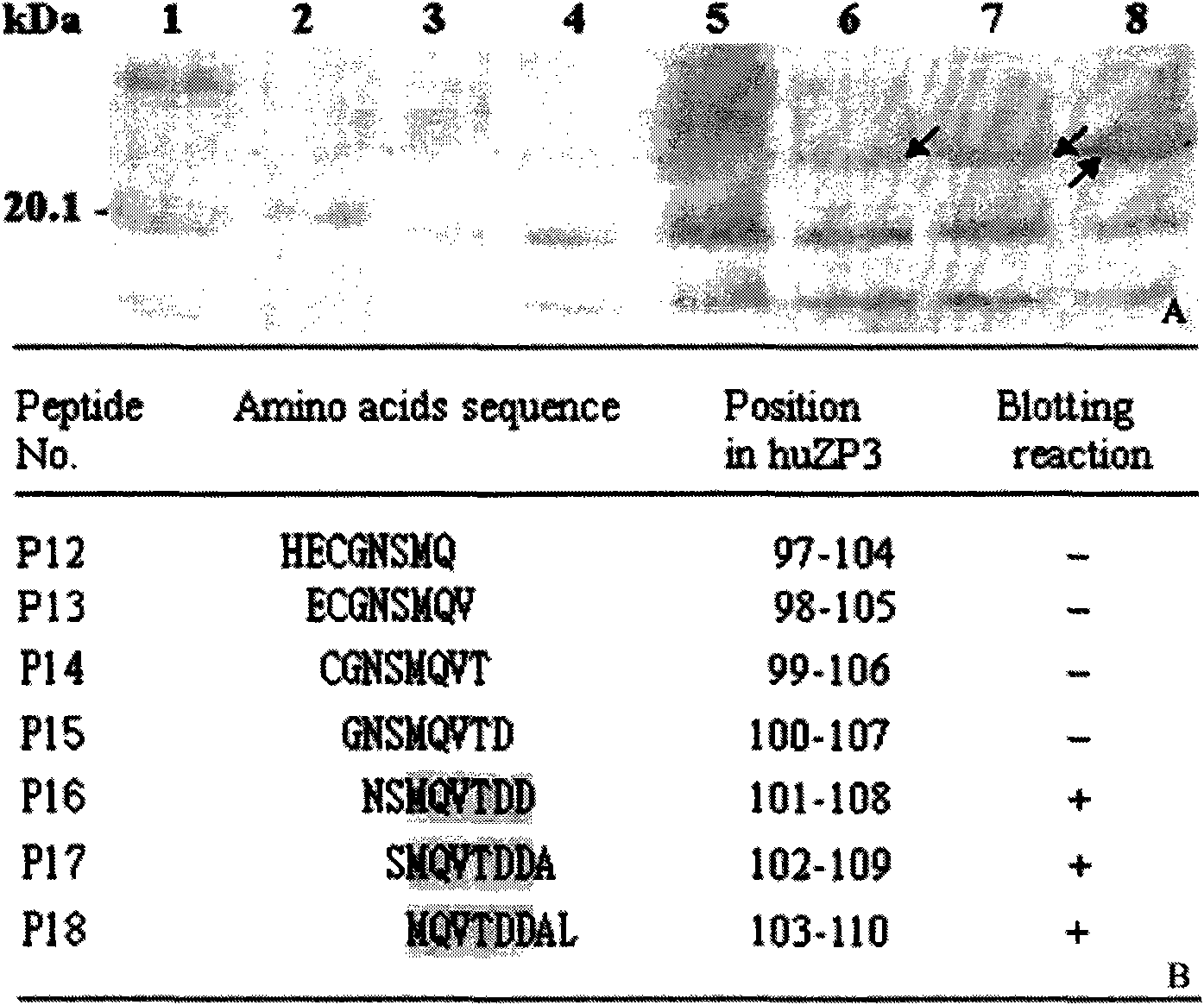
[0033] Example 2: Human egg zona pellucida protein-3 (huZP3) epitope 93-110 Minimal motif identification
[0034] Materials and methods: In addition to rabbit anti-huZP3a and huZP3b antiserum (prepared by the patent applicant. Song Liwen et al. Immunogenicity of human egg zona pellucida protein ZP3a and ZP3b peptides and their antisera inhibit human sperm-Zona translucency binding in vitro. Physiological Acta Sinica, 57:682-688, 2005) and horseradish peroxidase-labeled goat anti-human secondary antibody (IgG / HRP, purchased from PTGLAB Company), others see the corresponding part of Example 1.
[0035] wxya 93-110 The specific steps of epitope peptide minimal motif identification are as follows:
[0036]1. Based on the epitope identification and gene sequence public information (Paterson M, et al. Evaluation of the contraceptive potential recombinant human ZP3 and human ZP3 peptides in a primate model: their safety and efficacy. Am J Reprod Immunol, 40: 198-209, 1998; Chamber...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com